��Ŀ����
��9�֣�ij�о���ѧϰС�齫����װ����ͼ���ӣ�a��b��c��d��g ��h���Ƕ��Ե缫��A��ʢ��100mlCuSO4��Һ��B��ʢ��100g��������Ϊ16%������������Һ������Դ��ͨ����g�������Ժ�ɫ��
��h���Ƕ��Ե缫��A��ʢ��100mlCuSO4��Һ��B��ʢ��100g��������Ϊ16%������������Һ������Դ��ͨ����g�������Ժ�ɫ��

�Իش���������:
��1����ԴM����������______��
��2��ͨ��һ��ʱ�䣬ȡ���缫����A�м���4.9gCu(OH)2����Һ����ǰ��ͬ������ʱ��·��ͨ���ĵ���Ϊ_________mol��ԭCuSO4��Һ���ʵ���Ũ��Ϊ________mol?L-1��
(3)B��d�ĵ缫��ӦʽΪ________________________________________________��
d���ϲ������������Ϊ____________L(���)
(4)����Cװ�ø�ͭ������f��__________(������ͭ)�����Һ��__________��Һ��
(5)����D����Һ�����ձ���ϡ����200ml����ʱ��Һ��PH=_______��
 ��h���Ƕ��Ե缫��A��ʢ��100mlCuSO4��Һ��B��ʢ��100g��������Ϊ16%������������Һ������Դ��ͨ����g�������Ժ�ɫ��
��h���Ƕ��Ե缫��A��ʢ��100mlCuSO4��Һ��B��ʢ��100g��������Ϊ16%������������Һ������Դ��ͨ����g�������Ժ�ɫ��
�Իش���������:
��1����ԴM����������______��
��2��ͨ��һ��ʱ�䣬ȡ���缫����A�м���4.9gCu(OH)2����Һ����ǰ��ͬ������ʱ��·��ͨ���ĵ���Ϊ_________mol��ԭCuSO4��Һ���ʵ���Ũ��Ϊ________mol?L-1��
(3)B��d�ĵ缫��ӦʽΪ________________________________________________��
d���ϲ������������Ϊ____________L(���)
(4)����Cװ�ø�ͭ������f��__________(������ͭ)�����Һ��__________��Һ��
(5)����D����Һ�����ձ���ϡ����200ml����ʱ��Һ��PH=_______��

��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
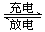 NiOOH + MH������������ȷ���ǣ� ��
NiOOH + MH������������ȷ���ǣ� ��
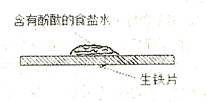
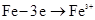

 ____��
____��
 O32����ʮc��OH����+c��HSO3����=c��Na+��+c��H+��
O32����ʮc��OH����+c��HSO3����=c��Na+��+c��H+��