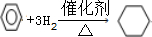
��Ŀ����
7����һ�����ļ���������Ļ����������װ�й������Ƶ��ܱ������У��õ��ȼ����ַ�Ӧ����1.01��105Pa��120��ʱ����������е�ѹǿ����Ϊ�㣬����Ӧ���ʣ�����Ͷ��ˮ��Ҳδ�������ݳ��֣��������ж��в���ȷ���ǣ�������| A�� | ��Ӧ��ʣ������ijɷ���̼���ƺ��������� | |
| B�� | ԭ����������м���������������2��1 | |
| C�� | ԭ����������м����������������Ϊ1��2 | |
| D�� | ԭ�����ܱ������У����顢�������������Ƶ����ʵ���֮��Ϊ2��1��6 |
���� ������O2��CH4�Ļ����������ʢ��23.4g Na2O2���ܱ������У����ȼ��ӦΪ��CH4+2O2=CO2+2H2O��2CO2+2Na2O2=2Na2CO3+O2��2H2O+2Na2O2=4NaOH+O2��
���������֪��Ӧ�����������ڵ�ѹǿΪ�㣨150�棩��˵�������������壬�ҽ�����������ˮ��������ų���˵����������Ҳ��ȫ��Ӧ����������ʣ�����ΪNa2CO3��NaOH�����ݼ�����������C��Hԭ���غ��֪��֪���ɵ�Na2CO3��NaOH���ʵ���֮��Ϊ1��4���ٸ��ݷ�Ӧ���ܻ�ѧ����ʽ�ж�ԭ������м��顢���������ʵ���֮�ȣ�
��� �⣺A����Ӧ��ѹǿΪ0pa��˵����Ӧ����������ʣ�����壬�ҽ�����������ˮ��������ų���˵����������Ҳ��ȫ��Ӧ����������ʣ�����ΪNa2CO3��NaOH����A��ȷ��
B�����ݼ���ķ�����ɣ�����C��Hԭ���غ��֪���ɵ�Na2CO3��NaOH���ʵ���֮��Ϊ1��4����Ӧ���ܻ�ѧ����ʽ��дΪ��2CH4+O2+6Na2O2=2Na2CO3+8NaOH��
���ݷ���ʽ2CH4+O2+6Na2O2=2Na2CO3+8NaOH��֪��ԭ���������O2��CH4�����ʵ���֮��Ϊ1��2����B��ȷ��
C�����ݼ���ķ�����ɣ�����C��Hԭ���غ��֪���ɵ�Na2CO3��NaOH���ʵ���֮��Ϊ1��4����Ӧ���ܻ�ѧ����ʽ��дΪ��2CH4+O2+6Na2O2=2Na2CO3+8NaOH��
���ݷ���ʽ2CH4+O2+6Na2O2=2Na2CO3+8NaOH��֪��ԭ���������O2��CH4�����ʵ���֮��Ϊ1��2����C����
D�����ݼ���ķ�����ɣ�����C��Hԭ���غ��֪���ɵ�Na2CO3��NaOH���ʵ���֮��Ϊ1��4����Ӧ���ܻ�ѧ����ʽ��дΪ��2CH4+O2+6Na2O2=2Na2CO3+8NaOH��
��֪��ԭ��������м��顢�������������Ƶ����ʵ���֮��Ϊ2��1��6����D��ȷ��
��ѡC��
���� ���⿼������ļ��㣬�Ѷ��еȣ��ؼ����ж�ʣ�����ΪNa2CO3��NaOH�����������غ㡢����ķ������ȷ��Na2CO3��NaOH���ʵ���֮�ȣ��������ܷ���ʽ������ʡȥ�м䷴Ӧ���Ӽ���Ĺ��̣�

| A�� | NaCl | B�� | MgCl2 | C�� | NaBr | D�� | NaI |
| �� | �� | �� | �� | �� | �� | �� | �� | �� | �� | |
| ԭ�Ӱ뾶/10-10m | 0.37 | 1.86 | 0.74 | 1.43 | 0.77 | 1.10 | 0.99 | 1.52 | 0.75 | 0.71 |
| ���̬ | +1 | +1 | +3 | +4 | +5 | +7 | +1 | +5 | ||
| ��ͼ�̬ | -1 | -2 | -4 | -3 | -1 | -3 | -1 |
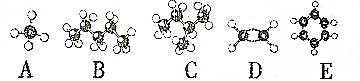
��2��Ԫ�آݡ��͢ߵ�ij����Ԫ���γɵĻ������У�ÿ��ԭ�Ӷ����������Ϊ8�����ȶ��ṹ��������CCl4��PCl3_��д��ѧʽ����
��3��ijԪ��R��ԭ�Ӱ뾶Ϊ1.02��10-10m���������γ�Na2R2�������ʽ��
 _��R��Ԫ�ط��ű�ʾ����
_��R��Ԫ�ط��ű�ʾ������4��Ԫ�آٺ͢��γ������ӣ���ṹʽΪ
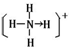 �����еĻ�ѧ�������ۼ�����λ����
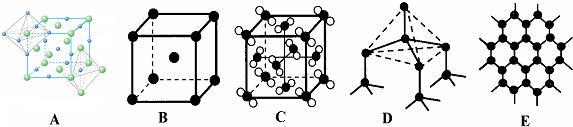
�����еĻ�ѧ�������ۼ�����λ������5��Ԫ�آں͢��γɾ���IJ��ֽṹ������ͼ�е�A_����ʾ������ţ���
| A�� | ���ȷֽⷨ������ʯ��ұ�������� | |
| B�� | �õ�������Ȼ�þ�ķ�����ȡ����þ | |
| C�� | ����ұ���Ĺ����ǽ��������仯�����л�ԭ���� | |
| D�� | �Ͼɽ����Ļ��������������ڼ�����Ⱦ�������ܺ� |
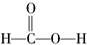 ���Լ���Ļ�ѧ���ʽ����������ƶϣ����в���ȷ���ǣ�������
���Լ���Ļ�ѧ���ʽ����������ƶϣ����в���ȷ���ǣ�������| A�� | ����̼������Һ��Ӧ | B�� | �ܷ���������Ӧ | ||
| C�� | ����ʹ����KMnO4��Һ��ɫ | D�� | ���뵥��þ��Ӧ |
| ʵ����� | ���� | ���� | |
| A | ʳ����ˮ�� | ������ɫ���� | ����������Ա�̼��ǿ |
| B | �Ҵ����ɫ�����ظ������Һ��� | ��ɫ��Һ��Ϊ��ɫ | �Ҵ����л�ԭ�� |
| C | ��Ƶε�����Ƭ�� | ����Ƭ���� | ����������̬����� |
| D | ����Cu��OH��2����������Һ��ϼ��� | ������ɫ���� | �����Ǿ��������� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | ���� | B�� | ����ϡ���ᣬ����98%Ũ���� | ||
| C�� | ��ˮϡ������ | D�� | ������Ƭ���������� |
 ��
��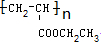 ��
��