��Ŀ����
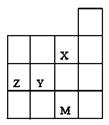
��14�֣��±�ΪԪ�����ڱ���һ���֣������Ԫ�آ�һ���ڱ��е�λ�ã��ش��������⣺
��1���ڡ��ۡ��ߵ���ۺ������������ǿ������˳���� ��д��ѧʽ����
��2���ɱ���Ԫ�ؿ�����ɶ���Ư������д���������ֳ���Ư���Ļ�ѧʽ�� �� ��
��3���١��ܡ�������Ԫ���γɵĻ������л�ѧ�������ͣ� ��
��4���ں͢��γɵĻ�������ܺ͢��γɵĻ�����֮�䷢��������ԭ��Ӧ��д���÷�Ӧ�Ļ�ѧ����ʽ�� ��
��5���ɱ���Ԫ���γɵij�������X��Y��Z��M��W�ɷ������·�Ӧ��
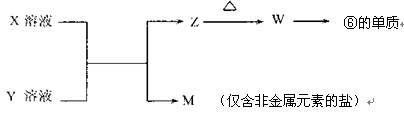
��X��Һ��Y��Һ��Ӧ�����ӷ���ʽΪ ��
����֪MΪ�����Σ���M��Һ�и�����Ũ���ɴ�С����˳��Ϊ��
c�� ��>c�� ��>c�� ��>c�� �����������������ӷ��ţ���
| �� ���� | I A | ��A | ��A | ��A | VA | ��A | ��A | 0 |
| һ | �� | | ||||||
| �� | | | | �� | �� | �� | | |
| �� | �� | | �� | �� | | �� | | |
��2���ɱ���Ԫ�ؿ�����ɶ���Ư������д���������ֳ���Ư���Ļ�ѧʽ�� �� ��
��3���١��ܡ�������Ԫ���γɵĻ������л�ѧ�������ͣ� ��
��4���ں͢��γɵĻ�������ܺ͢��γɵĻ�����֮�䷢��������ԭ��Ӧ��д���÷�Ӧ�Ļ�ѧ����ʽ�� ��
��5���ɱ���Ԫ���γɵij�������X��Y��Z��M��W�ɷ������·�Ӧ��

��X��Һ��Y��Һ��Ӧ�����ӷ���ʽΪ ��
����֪MΪ�����Σ���M��Һ�и�����Ũ���ɴ�С����˳��Ϊ��
c�� ��>c�� ��>c�� ��>c�� �����������������ӷ��ţ���
��1��HNO3>H2CO3>H2SiO3��2�֣�
��2��H2O2��SO2��Na2O2���Ⱥ����Ļ�ѧʽ�����֣�4�֣�
��3�����Ӽ������ۼ������Թ��ۼ�����2�֣�
��4��2Na2O2+2CO2=2Na2CO3+O2��2�֣�
��5����Al3++3NH3��H2O=Al��OH��3��+3NH4+��2�֣�
��c��NO3-��>c��NH4+��>c��H+��>c��OH-����2�֣�
��2��H2O2��SO2��Na2O2���Ⱥ����Ļ�ѧʽ�����֣�4�֣�
��3�����Ӽ������ۼ������Թ��ۼ�����2�֣�
��4��2Na2O2+2CO2=2Na2CO3+O2��2�֣�
��5����Al3++3NH3��H2O=Al��OH��3��+3NH4+��2�֣�
��c��NO3-��>c��NH4+��>c��H+��>c��OH-����2�֣�
��

��ϰ��ϵ�д�
�����Ŀ
 ��
��

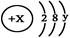 ��X��Y ��Ϊ������20�����������ݴ˻ش��������⣺
��X��Y ��Ϊ������20�����������ݴ˻ش��������⣺