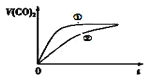
��Ŀ����
����Ŀ��ʯ���ǹ�ҵ��ѪҺ�������ǵ�����������ϢϢ��ء�
��1����ϩ��ʯ�ͻ�����Ҫ�Ļ���ԭ�ϡ� д����ϩ�ĵ���ʽ____________
��2������ʽΪC5H12��ij���������к���4�����������Ľṹ��ʽΪ_______________________��
��3������ϩ��Ϊͬϵ�����_______ ��(ѡ����)
a. CH3CH=CH2 b. CH2=CHCH=CH2
c. CH��CH d. CH3CH3
��4������ϩ��ȫ������������ʳƷ��װ��������ϩ�Ľṹ��ʽΪ ________________ ��
��5����Ȳ���ۿɵõ��������ϩ����Ȳ (CH2=CH-C-C-CH=CH2) �����𱽺Ͷ���ϩ����Ȳ���õ��Լ���__________________��
���𰸡��� 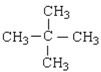 a
a ![]() ��ˮ�����Ը��������Һ
��ˮ�����Ը��������Һ
��������
������ϩΪ���ۻ������Cԭ�Ӻ�Hԭ�������ɴﵽ�ĵ�����д����ϩ�ĵ���ʽ��
�������ķ���ʽ�ͺ����ĸ���д���ṹ��ʽ��
����ͬϵ���������ƣ�����ʽ���n��CH2�����ѡ�������ʵĹ������жϣ�
���ݾ���ϩ������ϩͨ���Ӿ۵õ���д���ṹ��ʽ��
�ҳ��뱽����Ӧ�������ϩ����Ȳ��Ӧ������ɫ�����ʼ��ɼ�����������ʡ�
��1����ϩΪ���ۻ����Cԭ�������ɴﵽ8�����ӣ�Hԭ�������ɴﵽ2�����ӣ���ϩ��ȷ�ĵ���ʽΪ��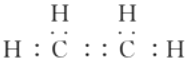 ��
��
��2���������ķ���ʽ�ͺ����ĸ�����֪������ֻ��Ϊ�����飬�ṹ��ʽΪ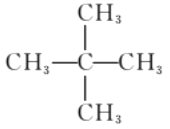 ��
��
��3������ͬϵ��Ķ����֪����ϩ��Ϊͬϵ���ֻ����a��
��4������ϩΪ��ϩͨ���Ӿ۷�Ӧ���ɵģ�����ȷ�Ľṹ��ʽΪ��![]() ��
��
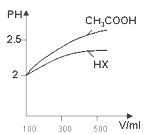
��5�����ݱ�������ˮ������������Һ����Ӧ������ϩ����Ȳ������ˮ������������Һ����Ӧ��ʹ��ˮ������������Һ����ɫ��֪������ˮ������������Һ�����𱽺Ͷ���ϩ����Ȳ�����н���ˮ������������Һ�����뵽����ʱ���ܹ۲쵽����������Һ�ֲ㣬�������ϣ�ˮ���£������ú���ʺ���ɫ��ˮ����ɫ��dz������Һ�ֲ㣬�������ϣ�ˮ���£������ú�����ɫ��ˮ���Գ���ɫ����

����Ŀ����ѧ������������ء�
I.K2Cr2O7�����ڼ��˾���Ƿ�ƺ��ʻ��Cr2O72-(��ɫ)+CH3CH2OH��Cr3+(��ɫ)+CH3COOH(δ��ƽ��
��1����̬Crԭ�ӵļ۵��ӹ������ʽΪ__��
��2��CH3COOH����������Ԫ�صĵ縺���ɴ�С��˳��Ϊ__��̼ԭ�ӵĹ���ӻ�����Ϊ__��������������������Ŀ֮��Ϊ__��
��3����֪Cr3+�ȹ���Ԫ��ˮ�����ӵ���ɫ�����ʾ��
���� | Sc3+ | Cr3+ | Fe2+ | Zn2+ |
ˮ�����ӵ���ɫ | ��ɫ | ��ɫ | dz��ɫ | ��ɫ |
�����ԭ�ӽṹ�Ʋ�Sc3+��Zn2+��ˮ������Ϊ��ɫ��ԭ��Ϊ__��
II.ZnCl2Ũ��Һ�����ڳ�ȥ��������������������FeO��Ӧ�ɵ�Fe[Zn(OH)Cl2]2��Һ��
��4��Fe[Zn(OH)Cl2]2��Һ�в����ڵ�������������__(��ѡ����ĸ)��
A.���Ӽ� B.���ۼ� C.������ D.��λ�� E.���»��� F.���
��Һ��[Zn(OH)Cl2]-�ĽṹʽΪ__��
III.п������������Ԫ��֮һ����ѻ���ʽ��ͼ1�������ṹ��ͼ2��
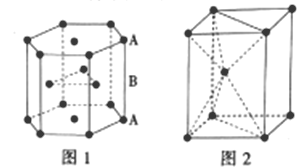
��5��п�Ķѻ���ʽΪ__����λ��Ϊ__��
��6����пԭ�ӵİ뾶Ϊapm�������ӵ�������ֵΪNA����п������ܶ�Ϊ___g/cm3(�ú�a�Ĵ���ʽ��ʾ)��
����Ŀ�����������ͻ����������ʵ��������������������һ����
��� | ʵ����������� | ���ͻ���� |
A | Ũ����ε�ֽ���ϣ�ֽ��� | Ũ��������ˮ�� |
B | ����ɫʯ����Һ�м�����ˮ����Һ�ȱ�죬�����ɫ | ��ˮ�к����������ʺ� Ư�������� |
C | ��ij��Һ�м���ϡ���ᣬ������ʹ����ʯ��ˮ����ǵ����� | ����Һ��һ����CO32- |
D | ��ij��Һ�м���ŨNaOH��Һ�����ȣ�������ʹʪ��ĺ�ɫʯ����ֽ���������� | ����Һ��һ������NH |
A.AB.BC.CD.D