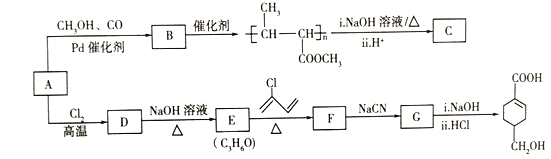
ЬтФПФкШн
ЁОЬтФПЁПA(C3H6)ЪЧЛљБОгаЛњЛЏЙЄдСЯЁЃгЩAжЦБИОлКЯЮяCКЭ![]() ЕФКЯГЩТЗЯп(ВПЗжЗДгІЬѕМўТдШЅ)ШчЭМЫљЪОЃК
ЕФКЯГЩТЗЯп(ВПЗжЗДгІЬѕМўТдШЅ)ШчЭМЫљЪОЃК
вбжЊЃК ЃЛ
ЃЛ![]() +R-COOH
+R-COOH
ЛиД№ЯТСаЮЪЬтЃК
(1)AЕФУћГЦЪЧ_______ЃЌBжаКЌбѕЙйФмЭХЕФУћГЦЪЧ_______________ЁЃ
(2)CЕФНсЙЙМђЪНЮЊ_______________ЃЌDЁњEЕФЗДгІРраЭЮЊ________________ЁЃ
(3)EЁњFЕФЛЏбЇЗНГЬЪНЮЊ____________________________ЁЃ
(4)![]() ЗЂЩњЫѕОлЗДгІЩњГЩгаЛњЮяЕФНсЙЙМђЪНЮЊ_________________ЁЃ
ЗЂЩњЫѕОлЗДгІЩњГЩгаЛњЮяЕФНсЙЙМђЪНЮЊ_________________ЁЃ
(5)BЕФЭЌЗжвьЙЙЬхжаЃЌгыBОпгаЯрЭЌЕФЙйФмЭХЧвФмЗЂЩњвјОЕЗДгІЕФЙВга________жжЃЛЦфжаКЫДХЙВеёЧтЦзЮЊ3зщЗхЃЌЧвЗхУцЛ§жЎБШЮЊ6ЃК1ЃК1ЕФЪЧ________________(аДНсЙЙМђЪН)ЁЃ
(6)НсКЯЬтИјаХЯЂЃЌвдввЯЉЁЂHBrЮЊЦ№ЪМдСЯжЦБИБћЫсЃЌЩшМЦКЯГЩТЗЯп(ЦфЫћЪдМСШЮбЁ)ЁЃ_________________ЁЃКЯГЩТЗЯпСїГЬЭМЪОР§ШчЯТЃКA![]() BЁЁ
BЁЁ![]() ФПБъВњЮяЁЃ
ФПБъВњЮяЁЃ
ЁОД№АИЁПБћЯЉ ѕЅЛљ  ШЁДњЗДгІЛђЫЎНтЗДгІ
ШЁДњЗДгІЛђЫЎНтЗДгІ 
![]() 8
8 ![]() CH2=CH2
CH2=CH2![]() CH3CH2Br
CH3CH2Br![]() CH3CH2CN
CH3CH2CN![]() CH3CH2COOH
CH3CH2COOH
ЁОНтЮіЁП
BЗЂЩњМгОлЗДгІЩњГЩОлЖЁЯЉЫсМзѕЅЃЌдђBНсЙЙМђЪНЮЊCH3CH=CHCOOCH3ЃЌAЮЊC3H6ЃЌAЗЂЩњМгГЩЗДгІЩњГЩBЃЌдђAНсЙЙМђЪНЮЊCH2=CHCH3ЃЌОлЖЁЯЉЫсМзѕЅЗЂЩњЫЎНтЗДгІШЛКѓЫсЛЏЕУЕНОлКЯЮяCЃЌCНсЙЙМђЪНЮЊ ЃЛAЗЂЩњЗДгІЩњГЩDЃЌDЗЂЩњЫЎНтЗДгІЩњГЩEЃЌEФмЗЂЩњЬтИјаХЯЂЕФМгГЩЗДгІЃЌНсКЯEЗжзгЪНжЊЃЌEНсЙЙМђЪНЮЊCH2=CHCH2OHЁЂDНсЙЙМђЪНЮЊCH2=CHCH2ClЃЌEКЭ2-ТШ-1ЃЌ3-ЖЁЖўЯЉЗЂЩњМгГЩЗДгІЩњГЩFЃЌFНсЙЙМђЪНЮЊ
ЃЛAЗЂЩњЗДгІЩњГЩDЃЌDЗЂЩњЫЎНтЗДгІЩњГЩEЃЌEФмЗЂЩњЬтИјаХЯЂЕФМгГЩЗДгІЃЌНсКЯEЗжзгЪНжЊЃЌEНсЙЙМђЪНЮЊCH2=CHCH2OHЁЂDНсЙЙМђЪНЮЊCH2=CHCH2ClЃЌEКЭ2-ТШ-1ЃЌ3-ЖЁЖўЯЉЗЂЩњМгГЩЗДгІЩњГЩFЃЌFНсЙЙМђЪНЮЊ![]() ЃЌFЗЂЩњШЁДњЗДгІЩњГЩGЃЌGЗЂЩњаХЯЂжаЗДгІЕУЕН
ЃЌFЗЂЩњШЁДњЗДгІЩњГЩGЃЌGЗЂЩњаХЯЂжаЗДгІЕУЕН ЃЌдђGНсЙЙМђЪНЮЊ
ЃЌдђGНсЙЙМђЪНЮЊ![]() ЃЛ
ЃЛ
ЃЈ1ЃЉЭЈЙ§вдЩЯЗжЮіжЊЃЌAЮЊБћЯЉЃЌBНсЙЙМђЪНЮЊCH3CH=CHCOOCH3ЃЌЦфКЌбѕЙйФмЭХУћГЦЪЧѕЅЛљЃЛ
ЃЈ2ЃЉCНсЙЙМђЪНЮЊ ЃЌDЗЂЩњЫЎНтЗДгІЛђШЁДњЗДгІЩњГЩEЃЌЙЪЗДгІРраЭЮЊШЁДњЗДгІЛђЫЎНтЗДгІЃЛ
ЃЌDЗЂЩњЫЎНтЗДгІЛђШЁДњЗДгІЩњГЩEЃЌЙЪЗДгІРраЭЮЊШЁДњЗДгІЛђЫЎНтЗДгІЃЛ
ЃЈ3ЃЉEЁњFЪЧCH2=CHCH2OHКЭ2-ТШ-1ЃЌ3-ЖЁЖўЯЉЗЂЩњМгГЩЗДгІЩњГЩ![]() ЃЌЗДгІЕФЛЏбЇЗНГЬЪНЮЊ
ЃЌЗДгІЕФЛЏбЇЗНГЬЪНЮЊ ЃЛ
ЃЛ
ЃЈ4ЃЉ![]() ЗЂЩњЫѕОлЗДгІВњЮяНсЙЙМђЪНЮЊ
ЗЂЩњЫѕОлЗДгІВњЮяНсЙЙМђЪНЮЊ![]() ЃЛ
ЃЛ
ЃЈ5ЃЉBНсЙЙМђЪНЮЊCH3CH=CHCOOCH3ЃЌBЕФЭЌЗжвьЙЙЬхжаЃЌгыBОпгаЯрЭЌЕФЙйФмЭХЧвФмЗЂЩњвјОЕЗДгІЃЌЫЕУїКЌгаЬМЬМЫЋМќКЭѕЅЛљЁЂШЉЛљЃЌЮЊМзЫсѕЅЃЌЗћКЯЬѕМўЕФЭЌЗжвьЙЙЬхгаHCOOCH=CHCH2CH3ЁЂHCOOCH2CH=CHCH3ЁЂHCOOCH2CH2CH=CH2ЁЂHCOOCЃЈCH3ЃЉ=CHCH3ЁЂHCOOCH=CЃЈCH3ЃЉ2ЁЂHCOOCЃЈCH3ЃЉCH=CH2ЁЂHCOOCHCЃЈCH3![]() ЃЛ
ЃЛ
ЃЈ6ЃЉCH2=CH2КЭHBrЗЂЩњМгГЩЗДгІЩњГЩCH3CH2BrЃЌCH3CH2BrКЭNaCNЗЂЩњШЁДњЗДгІЩњГЩCH3CH2CNЃЌCH3CH2CNдкМюадЬѕМўЯТЗЂЩњЫЎНтЗДгІШЛКѓЫсЛЏЕУЕНCH3CH2COOHЃЌЫљвдЦфКЯГЩТЗЯпЮЊCH2=CH2![]() CH3CH2Br
CH3CH2Br![]() CH3CH2CN
CH3CH2CN![]() CH3CH2COOHЁЃ
CH3CH2COOHЁЃ

ЁОЬтФПЁПЗМЯузхєШЫсЭЈГЃгУЗМЯуЬўЕФбѕЛЏРДжЦБИЁЃЗМЯуЬўЕФБНЛЗБШНЯЮШЖЈЃЌФбгкбѕЛЏЃЌЖјЛЗЩЯЕФжЇСДВЛТлГЄЖЬЃЌдкЧПСвбѕЛЏЪБЃЌзюжеЖМбѕЛЏГЩєШЛљЁЃФГЭЌбЇгУМзБНЕФбѕЛЏЗДгІжЦБИБНМзЫс ЁЃЗДгІдРэЃК
![]() +2KMnO4
+2KMnO4 ![]() +KOH+2MnO2 +H2O
+KOH+2MnO2 +H2O
![]() +HCl
+HCl![]() +KCl
+KCl
ЗДгІЪдМСЁЂВњЮяЕФЮяРэГЃЪ§ЃК
УћГЦ | ЯрЖдЗж згжЪСП | адзД | ШлЕу | ЗаЕу | УмЖШ | ШмНтЖШ | ||
ЫЎ | ввДМ | ввУб | ||||||
МзБН | 92 | ЮоЩЋвКЬхвзШМвзЛгЗЂ | -95 | 110.6 | 0.8669 | ВЛШм | взШм | взШм |
БНМзЫс | 122 | АзЩЋЦЌзДЛђеызДОЇЬх | 122.4 | 248 | 1.2659 | ЮЂШм | взШм | взШм |
жївЊЪЕбщзАжУКЭСїГЬШчЯТЃК
ЯТЭМЛиСїНСАшзАжУ

ЯТЭМГщТЫзАжУ

ЪЕбщЗНЗЈЃКвЛЖЈСПЕФМзБНКЭKMnO4ШмвКжУгкЭМ1зАжУжаЃЌдк90ЁцЪБЃЌ ЗДгІвЛЖЮЪБМфЃЌдйЭЃжЙЗДгІЃЌАДШчЯТСїГЬЗжРыГіБНМзЫсКЭЛиЪеЮДЗДгІЕФМзБНЁЃ

(1)ЮоЩЋвКЬхAЕФНсЙЙМђЪНЮЊ___________ЁЃВйзїЂђЮЊ__________ЁЃ
(2)ШчЙћТЫвКГЪзЯЩЋЃЌвЊЯШМгбЧСђЫсЧтМиЃЌШЛКѓдйМгШыХЈбЮЫсЫсЛЏЃЌМгбЧСђЫсЧтМиЕФФПЕФЪЧ______ЁЃ
(3)ЯТСаЙигквЧЦїЕФзщзАЛђепЪЙгУе§ШЗЕФЪЧ__________ЁЃ
AЃЎГщТЫПЩвдМгПьЙ§ТЫЫйЖШЃЌЕУЕННЯИЩдяЕФГСЕэ
BЃЎАВзАЕчЖЏНСАшЦїЪБЃЌНСАшЦїЯТЖЫВЛФмгыШ§ОБЩеЦПЕзЁЂЮТЖШМЦЕШНгДЅ
CЃЎЛиСїНСАшзАжУгІВЩгУжБНгМгШШЕФЗНЗЈ
DЃЎРфФ§ЙмжаЫЎЕФСїЯђЪЧЯТНјЩЯГі
(4)Г§ШЅВаСєдкБНМзЫсжаЕФМзБНгІЯШМгШы__________ЃЌЗжвКЃЌЫЎВудйМгШы__________ЃЌШЛКѓГщТЫЃЌИЩдяМДПЩЕУЕНБНМзЫсЁЃ
(5)ДПЖШВтЖЈЃКГЦШЁ1.220gВњЦЗЃЌХфГЩ100mlШмвКЃЌШЁЦфжа25.00mlШмвКЃЌНјааЕЮЖЈ ЃЌЯћКФKOHЮяжЪЕФСПЮЊ2.4ЁС10-3molЁЃВњЦЗжаБНМзЫсжЪСПЗжЪ§ЮЊ______________ЁЃ
ЁОЬтФПЁПНЋХЈЖШОљЮЊ0.01 mol/L ЕФH2O2ЁЂH2SO4ЁЂKIЁЂNa2S2O3ШмвКМАЕэЗлЛьКЯЃЌвЛЖЈЪБМфКѓШмвКБфЮЊРЖЩЋЁЃИУЪЕбщЪЧвЛжжЁАЕтжгЪЕбщЁБЁЃФГаЁзщЭЌбЇдкЪвЮТЯТЖдИУЁАЕтжгЪЕбщЁБЕФдРэНјааЬНОПЁЃ
ЃЈзЪСЯЃЉ
ИУЁАЕтжгЪЕбщЁБЕФзмЗДгІЃКH2O2 +2S2O32-+2H+=S4O62-+2H2O
ЗДгІЗжСНВННјааЃК
ЗДгІAЃКH2O2+2I-+2HЃЋ=I2+2H2O
ЗДгІBЃКЁЁ
(1)ЗДгІBЕФРызгЗНГЬЪНЪЧ______ЁЃЖдгкзмЗДгІЃЌI-ЕФзїгУЯрЕБгк______ЁЃ
(2)ЮЊжЄУїЗДгІAЁЂBЕФДцдкЃЌНјааЪЕбщЂёЁЃ
a.ЯђЫсЛЏЕФH2O2ШмвКжаМгШыЪдМСXЕФЫЎШмвКЃЌШмвКБфЮЊРЖЩЋЁЃ
b.дйЯђЕУЕНЕФРЖЩЋШмвКжаМгШыNa2S2O3ШмвКЃЌШмвКЕФРЖЩЋЭЪШЅЁЃ
ЪдМСXЪЧ______ЁЃ
(3)ЮЊЬНОПШмвКБфРЖПьТ§ЕФгАЯьвђЫиЃЌНјааЪЕбщЂђЁЂЪЕбщЂѓЁЃЃЈШмвКХЈЖШОљЮЊ0.01 mol/LЃЉ
ЪдМС ађКХ гУСПЃЈmLЃЉ | H2O2 ШмвК | H2SO4 ШмвК | Na2S2O3 ШмвК | KIШмвК (КЌЕэЗл) | H2O |
ЪЕбщЂђ | 5 | 4 | 8 | 3 | 0 |
ЪЕбщЂѓ | 5 | 2 | x | y | z |
ШмвКДгЛьКЯЪБЕФЮоЩЋБфЮЊРЖЩЋЕФЪБМфЃКЪЕбщЂђЪЧ30 minЁЂЪЕбщЂѓЪЧ40 minЁЃ
ЂйЪЕбщЂѓжаЃЌxЁЂyЁЂzЫљЖдгІЕФЪ§жЕЗжБ№ЪЧ______ЁЃ
ЂкЖдБШЪЕбщЂђЁЂЪЕбщЂѓЃЌПЩЕУГіЕФЪЕбщНсТлЪЧ______ЁЃ
(4)ЮЊЬНОПЦфЫћвђЫиЖдИУЁАЕтжгЪЕбщЁБЕФгАЯьЃЌНјааЪЕбщЂєЁЃ
ЃЈШмвКХЈЖШОљЮЊ0.01 mol/LЃЉ
ЪдМС ађКХ гУСПЃЈmLЃЉ | H2O2 ШмвК | H2SO4 ШмвК | Na2S2O3 ШмвК | KIШмвК(КЌЕэЗл) | H2O |
ЪЕбщЂє | 4 | 4 | 9 | 3 | 0 |
ЪЕбщЙ§ГЬжаЃЌШмвКЪМжеЮоУїЯдбеЩЋБфЛЏЁЃ
ЪдНсКЯИУЁАЕтжгЪЕбщЁБзмЗДгІЗНГЬЪНМАЗДгІAгыЗДгІBЫйТЪЕФЯрЖдПьТ§ЙиЯЕЃЌНтЪЭЪЕбщЂєЮДВњЩњбеЩЋБфЛЏЕФдвђЃК_____________________ЁЃ

