��Ŀ����
14��2015��8��12��23��30���ң�������������һ����װ����ͷ������ը��������ը���Ǽ�װ���ڵ���ȼ�ױ���Ʒ�軯�ƣ�����Ϊ700�����ң����ϣ��軯�ƻ�ѧʽΪNaCN����ɫ�ᾧ�������ĩ���׳��⣬�����Ŀ�������ζ���綾��Ƥ���˿ڽӴ������롢��ʳ�����ж��������۵�563.7�棬�е�1496�森������ˮ����ˮ�������軯�⣬ˮ��Һ��ǿ���ԣ���һ����Ҫ�Ļ���ԭ�ϣ����ڵ�ơ�ұ����л��ϳ�ҽҩ��ũҩ�������������森
��1�������ӷ���ʽ��ʾ��ˮ��Һ��ǿ���Ե�ԭ��CN-+H2O?HCN+OH-��
��2���軯��Ҫ��˫��ˮ������������кͣ�����˫��ˮ��������һ����ʽ�κ�һ����ʹʪ��ĺ�ɫʯ����ֽ���������壬��д���÷�Ӧ�Ļ�ѧ����ʽNaCN+H2O2+H2O�TNaHCO3+NH3����
��������������к͵����ӷ���ʽΪCN-+S2O32-�TA+SO32-��AΪSCN-���ѧʽ����
��3�������ˮ�е�CN-�о綾��
��CN-��CԪ����+2�ۣ�NԪ����-3�ۣ���ǽ�����N��C�����=����
��������������£�CN-�ܹ�������������HCO3-��ͬʱ����NH3���÷�Ӧ�����ӷ���ʽΪ2CN-+4H2O+O2$\frac{\underline{\;����\;}}{\;}$2HCO3-+2NH3��
����ͼ1��ʾװ�ó�ȥ��CN-��Cl-��ˮ�е�CN-ʱ��������ҺPHΪ9��10������������ClO-��CN-����Ϊ��������Ⱦ�����壬����˵������ȷ����
A����ʯī����������������
B�������ĵ缫��ӦʽΪ��Cl-+2OH--2e-�TClO-+H2O
C�������ĵ缫��ӦʽΪ��2H2O+2e-�TH2��+2OH-
D����ȥCN-�ķ�Ӧ��2CN-+5ClO-+2H+�TN2��+2CO2��+5Cl-+H2O
��4����̼���ƣ�2Na2CO3•3H2O2����һ�ּ�ϴ�ӡ�Ư�ס�ɱ����һ�����ϵƯ����Ҳ�����ں����ˮ��������ij��ȤС���Ʊ���̼���Ƶ�ʵ�鷽����װ��ʾ��ͼ��ͼ2
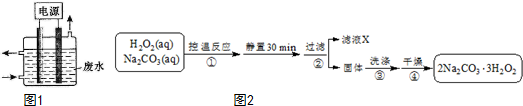
��֪��2Na2CO3 ��aq��+3H2O2 ��aq��?2Na2CO3•3H2O2 ��s����H��0����ش��������⣺
�����������У����������̼���Ʒ���������ԭ��Ӧ����C��
A��FeCl3B��CuSO4C��Na2SiO3D��KCN
��ȷ��ȡ0.2000g��̼������250mL��ƿ�У���50mL����ˮ�ܽ⣬�ټ�50mL2.0mol•L-1 H2SO4����0.02000mol•L-1 KMnO4 ����Һ�ζ����յ�ʱ����30.00mL�����Ʒ��H2O2����������Ϊ25.50%��[��Ӧ6KMnO4+5��2Na2CO3•3H2O2��+19H2SO4�T3K2SO4+6MnSO4+10Na2SO4+10CO2��+15O2��+34H2O]��
���� ��1���軯��Ϊǿ�������Σ�ˮ��������������������ƣ���Һ�ʼ��ԣ�
��2����ʹʪ��ĺ�ɫʯ����ֽ����������Ϊ����������ԭ���غ㣬ͬʱ����̼�����ƣ�
�ڸ���ԭ���غ�͵���غ���
��3���ٷǽ�����ǿ��Ԫ����ʾ���ۣ��ǽ�������������ʾ���ۣ�
�ڸ�����Ϣ��CN-�ܹ�������������HCO3-��ͬʱ����NH3����д����ʽ��
��A���õ������Һ�ʼ��ԣ����ʱ���ò����ý�����ǽ����������������ýϲ����ý�����������
B��������������ʧ�����������������������������ӷ�Ӧ���ɴ���������Ӻ�ˮ��
C��������ˮʧ�����������������������ӣ�
D������������ClO-��CN-����Ϊ��������Ⱦ�����壬�÷�Ӧ�ڼ��������½��У�����Ӧ�����������������ɣ�
��4��˫��ˮ��̼���ƻ�Ͽ����¶ȷ�����Ӧ2Na2CO3 ��aq��+3H2O2 ��aq��?2Na2CO3•3H2O2��s�������ù��˵õ�����2Na2CO3•3H2O2��������ϴ�ӡ�����õ��ϴ�����2Na2CO3•3H2O2��
�ٹ�̼�����൱�ڴ��ᾧ˫��ˮ��̼���ƣ�����˫��ˮ�����ʣ���������ԭ�������״ٽ���̼���Ʒ�Ӧ������ʧЧ��
����д����Ӧ�Ļ�ѧ����ʽ��Ȼ�����˫��ˮ�������ص����ʵ�����ϵ�������Ʒ��˫��ˮ�İٷֺ�����
��� �⣺��1���軯��Ϊǿ�������Σ�ˮ�ⷴӦΪ��CN-+H2O?HCN+OH-����Һ�ʼ��ԣ�
�ʴ�Ϊ��CN-+H2O?HCN+OH-��
��2������˫��ˮ�����軯�ƣ�����һ����ʹʪ��ĺ�ɫʯ����ֽ����������Ϊ����������ԭ���غ�һ����ʽ��Ϊ̼�����ƣ����Է�ӦΪ��NaCN+H2O2+H2O�TNaHCO3+NH3����
�ʴ�Ϊ��NaCN+H2O2+H2O�TNaHCO3+NH3����
��CN-+S2O32-�TA+SO32-�����ݵ���غ㣬AΪ-1�۵������ӣ�����ԭ���غ㣬A�к���1����ԭ�ӡ�1��̼ԭ�ӡ�1����ԭ�ӣ�����AΪ��SCN-��
�ʴ�Ϊ��SCN-��
��3����CN-��CԪ����+2�ۣ�NԪ����-3�ۣ�˵��N�ǽ�����ǿ��
�ʴ�Ϊ������
��CN-�ܹ�������������HCO3-��ͬʱ����NH3�ó�����ʽΪ��2CN-+4H2O+O2$\frac{\underline{\;����\;}}{\;}$2HCO3-+2NH3��
�ʴ�Ϊ��2CN-+4H2O+O2$\frac{\underline{\;����\;}}{\;}$2HCO3-+2NH3��
��A���õ������Һ�ʼ��ԣ����ʱ���ò����ý�����ǽ����������������ýϲ����ý��������������Կ�����ʯī����������������������A��ȷ��
B��������������ʧ�����������������������������ӷ�Ӧ���ɴ���������Ӻ�ˮ������������ӦʽΪCl-+2OH--2e-�TClO-+H2O����B��ȷ��
C���������Һ�ʼ��ԣ���������ˮʧ�����������������������ӣ��缫��ӦʽΪ2H2O+2e-�TH2��+2OH-����C��ȷ��
D������������ClO-��CN-����Ϊ��������Ⱦ�����壬��������Ϊ������̼�͵������÷�Ӧ�ڼ��������½��У�����Ӧ�����������������ɣ���Ӧ����ʽΪ2CN-+5ClO-+H2O�TN2��+2CO2��+5Cl-+2OH-����D����
�ʴ�Ϊ��D��
��4���ٹ�̼�����൱�ڴ��ᾧ˫��ˮ��̼���ƣ�����˫��ˮ�����ʣ�FeCl3����˫��ˮ�ֽ�Ĵ�����KCN���л�ԭ�ԣ��ܱ���̼����������CuSO4��˫��ˮ�ķֽ⣬�����Ʋ����̼���Ʒ�Ӧ��
�ʴ�Ϊ��C��
�ڸ��������Һ���̼���Ʒ�Ӧ�Ļ�ѧ����ʽΪ��6KMnO4+5��2Na2CO3•3H2O2��+19H2SO4�T3K2SO4+6MnSO4+10Na2SO4+10CO2��+15O2��+34H2O���ζ�����������2.000x10-2 mol•L-1 KMnO4����Һ�����ʵ���Ϊ��2.000x10-2 mol•L-1��0.03L=6.000x10-4mol��
��������������Ϊxg�����ݹ�ϵʽ
KMnO4��2Na2CO3•3H2O2��15H2O2
6mol 15��34g
6.000x10-4mol x
x=0.051
�����������������=$\frac{0.051g}{0.2g}$=25.50%
�ʴ�Ϊ��25.50%��
���� ���⿼���˹�̼���Ƶ�ʵ�鷽������ѧ����ʽ��д���������������ļ����֪ʶ����Ŀ�ѶȽϴ�ע�⻯ѧʵ��ԭ�����ܹ����ݷ�Ӧ����ʽ���мĻ�ѧ���㣬����������ѧ���ķ������������������Ӧ����ѧ֪ʶ��������

| A�� | ���ᱵ������ˮ���������ᱵ���ǵ���� | |
| B�� | ������̼����ˮ���Ե��磬���Զ�����̼�ǵ���� | |
| C�� | ��̬�����ǵ���ʣ���������������ʱ������ˮʱ���ܵ��� | |
| D�� | Һ̬���ܵ��磬�������ǵ���� |
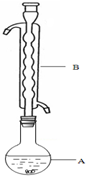 Na2S2O3�׳ƴ��մ���������Ҫ�Ļ���ԭ�ϣ���Na2SO3�������ˮ��Һ�м��ȷ�Ӧ�������Ƶ�Na2S2O3����֪10���70��ʱ��Na2S2O3��100gˮ�е��ܽ�ȷֱ�Ϊ60.0g��212g�������£�����Һ�������ľ�����Na2S2O3•5H2O����ʵ��������ȡNa2S2O3•5H2O���壨Na2S2O3•5H2O�ķ�����Ϊ248���������£�
Na2S2O3�׳ƴ��մ���������Ҫ�Ļ���ԭ�ϣ���Na2SO3�������ˮ��Һ�м��ȷ�Ӧ�������Ƶ�Na2S2O3����֪10���70��ʱ��Na2S2O3��100gˮ�е��ܽ�ȷֱ�Ϊ60.0g��212g�������£�����Һ�������ľ�����Na2S2O3•5H2O����ʵ��������ȡNa2S2O3•5H2O���壨Na2S2O3•5H2O�ķ�����Ϊ248���������£��ٳ�ȡ12.6g Na2SO3���ձ��У�����80.0mLˮ��
����ȡ4.0g��ۣ��������Ҵ���ʪ�ӵ�������Һ�У�
�ۣ���ͼ��ʾ������װ����ȥ����ˮԡ���ȣ��У���ӦԼ1Сʱ����ˣ�
����Һ�ھ�������Ũ������ȴ�ᾧ������Na2S2O3•5H2O���壮
�ݽ��м�ѹ���˲����
��1������B�����������������ܣ������������������������������Ҵ���ʪ��Ŀ�������ӷ�Ӧ��Ӵ��������߷�Ӧ���ʣ�
��2�������Ӧ��ȡ�IJ���������Ũ������ȴ�ᾧ��
��3����Һ�г�Na2S2O3�Ϳ���δ��Ӧ��ȫ��Na2SO3�⣬����ܴ��ڵ���������Na2SO4�������Һ�и����ʵĺ������ܵͣ�����ķ����ǣ�ȡ��������Һ����ϡ���������ԣ����ú�ȡ�ϲ���Һ����˳�ȥS���ټ�BaCl2��Һ�������ֻ�����Na2SO4����֮������
��4��Ϊ�˲��Ʒ�Ĵ��ȣ���ȡ7.40g ��Ʒ�����Ƴ�250mL��Һ������Һ����ȡ25��00mL����ƿ�У��μӵ�����Һ��ָʾ��������Ũ��Ϊ0.0500mol/L �ĵ�ˮ������ʽ�����ʽ����ʽ�����ζ������ζ���2S2O32-+I2=S4O62-+2I-�����ζ�������£�
| �ζ����� | �ζ�ǰ������mL�� | �ζ��ζ��������mL�� |
| ��һ�� | 0.30 | 31.12 |
| �ڶ��� | 0.36 | 31.56 |
| ������ | 1.10 | 31.88 |
| A�� | �������Ƶ���ˮʱ����Һ��pH��С | |
| B�� | ���¸�ѹ������N2��H2ת��ΪNH3��N2��g��+3H2��g��?2NH3��g������H��0�� | |
| C�� | ����ˮƿʱ���д��������ݳ� | |
| D�� | ��ˮӦ�ܱձ����ڵ��´� |
 ��Һ������ɫ��30s�ڲ���ɫ
��Һ������ɫ��30s�ڲ���ɫ