��Ŀ����
����˵���У�����ȷ���ǣ��� ��
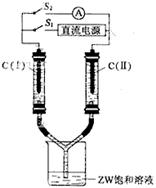
| A����ⱥ��ʳ��ˮ�������Ȼ���ʱ�������ĵ缫��Ӧʽ��Ϊ��2Cl - ��2e -=Cl2 �� |
| B�����Խ��ʻ���Խ��ʵ�����ȼ�ϵ�ص�������Ӧʽ��Ϊ��O2 + 2H2O+ 4e- = 4OH - |
| C������ͭ�͵��ͭʱ�����Դ���������ĵ缫��Ӧʽ��Ϊ�� Cu2+ + 2e- =" Cu" |
| D����������������ʴ�����ⸯʴ�ĸ�����Ӧʽ��Ϊ��Fe��2e - = Fe2+ |
B
�������������������ʧȥ���ӣ�����������Ӧ�����������ӵķŵ�����ǿ��OH���ģ����Ե�ⱥ��ʳ��ˮ�������Ȼ���ʱ�������ĵ缫��Ӧʽ��Ϊ��2Cl - ��2e -��Cl2����A��ȷ��B����ȷ�������Խ����У������缫��Ӧʽ��O2 + 4H�� + 4e- �� 2H2O�����������Դ�������������������õ����ӣ�������ԭ��Ӧ�����Ծ���ͭ�͵��ͭʱ�����Դ���������ĵ缫��Ӧʽ��Ϊ�� Cu2+ + 2e- �� Cu��ѡ��C��ȷ���ڸ����ĵ绯ѧ��ʴ�У��������ڸ���ʧȥ���ӣ�����������ѡ��D��ȷ����ѡB��
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣����Ĺؼ�����ȷԭ��غ͵��صĹ���ԭ�����ر��ǵ缫��Ӧʽ���жϡ����ӵķŵ�˳��Ȼ��������������⡢����������ɡ������ڼ���ѧ����ѧϰ��Ȥ����ǿѧ����ѧϰ������

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ

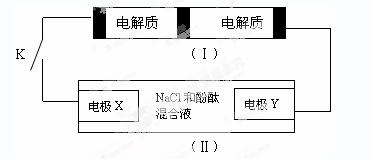
 Na2S4+3NaBr�����պϿ���Kʱ��X�缫������Һ��졣����˵����ȷ����
Na2S4+3NaBr�����պϿ���Kʱ��X�缫������Һ��졣����˵����ȷ����