��Ŀ����
��ˮռ�����ܴ�ˮ����97.2%�����Ѻ�ˮ�����ͻ�����������������ȿ��Խ����ˮ��Դȱ�������⣬�ֿ��Գ�����ú�����Դ��
��1����ˮ�к��д������Ȼ��ƣ��Ȼ����еĽ���Ԫ��λ��Ԫ�����ڱ���__________�塣
��2��Ŀǰ������ʹ�õġ���ˮ��������Ҫ����֮һ�����������ǽ���ˮ�������������������ȴ���øߴ��ȵ�ˮ���ɴ˿��ж�������____________���������仯��ѧ�仯����
��3����ҵ�����õ�ⱥ��ʳ��ˮ���Ƶ���Ҫ������Ʒ����ӦʽΪʳ��+H2O![]() NaOH+H2+Cl2(δ��ƽ�����÷�Ӧ��ʳ�εĻ�ѧʽ��____________�����õ������������36.5%��Ũ����1 000 t��������Ҫ����ʳ��____________t��
NaOH+H2+Cl2(δ��ƽ�����÷�Ӧ��ʳ�εĻ�ѧʽ��____________�����õ������������36.5%��Ũ����1 000 t��������Ҫ����ʳ��____________t��
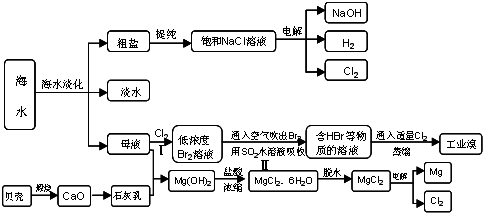
��4�����������������һ�������ȼҵ��Ʒ���Ȼ���ѭ������������������������ն�������ķ������÷������������£�

д���ڢܵĻ�ѧ��Ӧ����ʽ��____________________________________________��
�����������������ȩ����һ����Ӧ���Ժϳ��׳ơ����飨��ۣ��������ʡ������������к�����������ʳƷƯ�ס���ṹ��ʽΪHOCH2SO3Na��������ṹ�ص㣬�����ܷ����ķ�Ӧ��____________��
A.������Ӧ����
B.�ۺϷ�Ӧ
C.������Ʒ�Ӧ����
D.������Ӧ
��1����A?
��2�������仯?
��3��NaCl��585?
��4��NaOH+SO2====NaHSO3��HCl+NaHSO3====NaCl+H2O+SO2��?
��5��CD?
����:
��1��NaԪ��λ�ڵ�3���ڡ��ڢ�A�壬ע�ⲻ��д�ɵ�һ�塣������ͨ������ʹˮ��״̬�����仯���������仯����ⱥ��ʳ��ˮ�ķ�ӦΪ2NaCl+2H2O====2NaOH+H2��+Cl2����������H2��Cl2��Ӧ��H2+Cl2![]() 2HCl���ۺ�������Ӧ�ã�NaCl��HCl����ҪNaCl������Ϊ
2HCl���ۺ�������Ӧ�ã�NaCl��HCl����ҪNaCl������Ϊ![]() ��58.5 g��mol-1=585 t�������顱�к��С�OH���ǻ������������Na��Ӧ��Ҳ�ܷ���������Ӧ��
��58.5 g��mol-1=585 t�������顱�к��С�OH���ǻ������������Na��Ӧ��Ҳ�ܷ���������Ӧ��