��Ŀ����
����Ŀ����������ʮ�����ʣ���H2 ���� �۴��� ��CO2 ��H2SO4 ��Ba(OH)2���� �߰�ˮ ��ϡ���������Al2(SO4)3 ��NaHSO4��
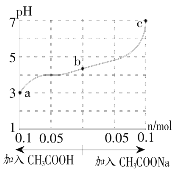
(1)�����ʵķ������д����Ŀհ״���
���ڷǵ���ʵ���__________�����ڵ���ʵ���__________���ܵ������____________��
(2)����ʮ������������������֮��ɷ������ӷ�Ӧ��H++OH===H2O�������ӷ�Ӧ��Ӧ�Ļ�ѧ����ʽΪ_____________________________��
(3)д���ۺ͢߷�Ӧ�����ӷ���ʽΪ__________________��34.2 g ������ˮ���250 mL��Һ��SO42-�����ʵ���Ũ��Ϊ_________________��
(4)�����Ģ�ͨ�����Һ�з�Ӧ�����ӷ���ʽΪ______________________��
(5)�������Һ�������Һ������Һ�е�Ba2+����ǡ����ȫ����ʱ��Ӧ�����ӷ���ʽΪ__________��
���𰸡��ܢۢݢޢ��ڢߢ��Ba(OH)2+2HNO3=Ba(NO3)2+2H2OCH3COOH+NH3��H2O=CH3COO+NH4++H2O1.20 mol/LCO2+OH=HCO3-2H++SO42-+Ba2++2OH= BaSO4��+2H2O
��������
(1). CO2 ����Һ���������ܵ��룬���ڷǵ���ʣ����ᡢ���������ᣬBa(OH)2���ڼAl2(SO4)3��NaHSO4�����Σ���ˮ��Һ�ж����Ե��������ʹ��Һ���е����ԣ����ڵ���ʣ����ǽ��������Ե��磬��ϡ������Һ�У��������������Ӻ���������ӣ����Ե��磬�ڰ�ˮ��Һ�У�һˮ�ϰ������笠����Ӻ����������ӣ����Ե��磬����Al2(SO4)3����������Ӻ���������ӣ����Ե��磬�ʴ�Ϊ���ܣ��ۢݢޢ�⣻�ڢߢ��
(2.)����������������Ӧ��ʵ���������������������ӷ�Ӧ����ˮ�����������ӷ���ʽH++OH=H2O��ʾ����Ӧ��Ӧ����ʽΪ��Ba(OH)2+2HNO3=Ba(NO3)2+2H2O���ʴ�Ϊ��Ba(OH)2+2HNO3=Ba(NO3)2+2H2O��
(3). �����һˮ�ϰ���ˮ��Һ�ж�������ȫ���룬���Դ���Ͱ�ˮ��Ӧ�����ӷ���ʽΪCH3COOH+NH3H2O=CH3COO+NH4++H2O��34.2 g �����������ʵ���Ϊ![]() =0.10mol�����������Ļ�ѧʽ��֪����������ӵ����ʵ���Ϊ0.30mol������ˮ���250 mL��Һ��SO42-�����ʵ���Ũ��Ϊc(SO42-)=
=0.10mol�����������Ļ�ѧʽ��֪����������ӵ����ʵ���Ϊ0.30mol������ˮ���250 mL��Һ��SO42-�����ʵ���Ũ��Ϊc(SO42-)=![]() =1.20mol/L���ʴ�Ϊ��CH3COOH+NH3H2O=CH3COO+NH4++H2O��1.20mol/L��
=1.20mol/L���ʴ�Ϊ��CH3COOH+NH3H2O=CH3COO+NH4++H2O��1.20mol/L��
(4).Ba(OH)2��Һ�������CO2��Ӧ����̼���Ⱶ���÷�Ӧ�����ӷ���ʽΪ��OH+CO2= HCO3-���ʴ�Ϊ��OH+CO2= HCO3-��
(5).���������Ƶ���Һ����Ba(OH)2��Һ������Һ�е�Ba2+����ǡ����ȫ����ʱ��Ba(OH)2��NaHSO4�����ʵ���֮��Ϊ1:2���÷�Ӧ�����ӷ���ʽΪ��2H++SO42+Ba2++2OH=BaSO4��+2H2O���ʴ�Ϊ��2H++SO42+Ba2++2OH=BaSO4��+2H2O��

����Ŀ�����к͵ζ����ⶨij�ռ�Ĵ��ȣ��Ը���ʵ��ش�
��1����ȡ4.1g�ռ���Ʒ������Ʒ���250mL����Һ����Ҫ����Ҫ�����������ձ������������__________________ ��______________________��
��2��ȡ10.00mL����Һ����___________________��ȡ��
��3����0.2010mol��L-1������ζ������ռ���Һ���Է�̪Ϊָʾ�����ζ�ʱ������ת��ʽ�ζ��ܵIJ������������ֲ�ͣ��ҡ����ƿ������ע��_____________��ֱ������______________________________�����жϴﵽ�ζ��յ㡣
��4�������������ݣ���������ռ���Һ��Ũ��Ϊ��_____________________�����������λ��Ч���֣�����Ʒ�ռ����������Ϊ________________�����������λ��Ч���֣���(�����ռ��в��������ᷴӦ������)
�ζ����� | ����Һ��� (mL) | ���������(mL) | |
�ζ�ǰ����(mL) | �ζ������(mL) | ||
��һ�� | 10.00 | 0.50 | 20.40 |
�ڶ��� | 10.00 | 4.00 | 24.10 |
��5���ζ����������������ʹ�ⶨ���ƫ�ߵ���_____________________________����ţ���
����ʽ�ζ�����ˮϴ���װҺ����еζ����ڼ�ʽ�ζ���ˮϴ��������ȡ����Һ������ƿ������ˮϴ�Ӻ����ô���Һ��ϴ���ܵζ������ϸ��������Һ������ƿ�ڶ���δҡ��ϴ�£��������ڵζ�ʱ������ƿ�⣻�μ����ᣬ��Һ��ɫ��ȥ�����������ָֻ���ɫ���ߵζ�ǰ����ʽ�ζ��������ݣ��ζ�����ʧ�����¼��ʼ���ʱ�����Ӷ������յ�ʱ���ӡ�
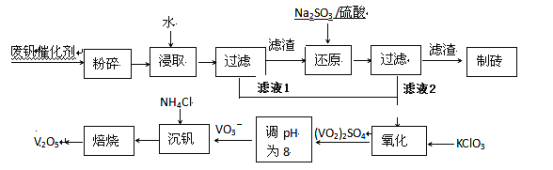
����Ŀ�����Ų��Ͽ�ѧ�ķ�չ�����������仯����õ���Խ��Խ�㷺��Ӧ�ã�������Ϊ���Ͻ��ά���ء���Ϊ�������ú�������������V2O5��VOSO4�������Բ�������������Ա����������һ�����ӽ��������շ����¹��գ������ʴ�91.7%���ϡ����ֺ���������ˮ�е��ܽ������±���ʾ��
���� | VOSO4 | V2O5 | NH4VO3 | (VO2)2SO4 |
�ܽ��� | ���� | ���� | ���� | ���� |
�ù��յ���Ҫ�������¡�
��ش��������⣺
��1����д������Na2SO3��Һ������Ӧ�����ӷ���ʽ_________��
��2����������ʹ�õĴ�������ý(V2O5)�ܼӿ���������������ʣ��˹����в�����һ�������м��壨����ͼ��������a��c�����Ļ�ѧ����ʽ�ɱ�ʾΪ_________________��______________��
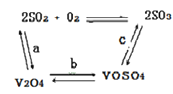
��3���ù����г������ǻ��շ��Ĺؼ�֮һ�������ʵĸߵͳ�����ҺpHӰ���⣬����Ҫ�����Ȼ��ϵ����NH4Cl������������Һ��V2O5�������ȣ����¶ȡ�������ͼ�Խ�������Ȼ��ϵ��Ϊ_________�������¶ȵķ���Ϊ_________________��

��4������Һ1����Һ2��Ϻ����������������Ԫ�ر���ԭΪ��ͼۣ��䷴Ӧ�����ӷ�Ӧ����ʽΪ___��
��5���������ط�����ã�NH4VO3�ڱ��չ����У����������ļ���ֵ�������꣩���¶ȱ仯��������ͼ��ʾ����NH4VO3�ڷֽ������_________��

A���ȷֽ�ʧȥH2O���ٷֽ�ʧȥNH3 B���ȷֽ�ʧȥNH3���ٷֽ�ʧȥH2O
C��ͬʱ�ֽ�ʧȥH2O��NH3D��ͬʱ�ֽ�ʧȥH2��N2��H2O
��6��ȫ����صĵ������ҺΪVOSO4��Һ����صĹ���ԭ��ΪVO2+ + V2++2H+ ![]() VO2+ +H2O +V3+����س��ʱ�����ĵ缫��ӦʽΪ___________��
VO2+ +H2O +V3+����س��ʱ�����ĵ缫��ӦʽΪ___________��