��Ŀ����
����Ŀ�����Ų��Ͽ�ѧ�ķ�չ�����������仯����õ���Խ��Խ�㷺��Ӧ�ã�������Ϊ���Ͻ��ά���ء���Ϊ�������ú�������������V2O5��VOSO4�������Բ�������������Ա����������һ�����ӽ��������շ����¹��գ������ʴ�91.7%���ϡ����ֺ���������ˮ�е��ܽ������±���ʾ��
���� | VOSO4 | V2O5 | NH4VO3 | (VO2)2SO4 |
�ܽ��� | ���� | ���� | ���� | ���� |
�ù��յ���Ҫ�������¡�

��ش��������⣺
��1����д������Na2SO3��Һ������Ӧ�����ӷ���ʽ_________��
��2����������ʹ�õĴ�������ý(V2O5)�ܼӿ���������������ʣ��˹����в�����һ�������м��壨����ͼ��������a��c�����Ļ�ѧ����ʽ�ɱ�ʾΪ_________________��______________��
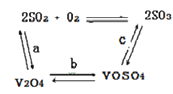
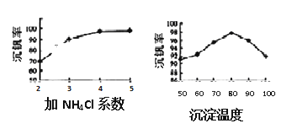
��3���ù����г������ǻ��շ��Ĺؼ�֮һ�������ʵĸߵͳ�����ҺpHӰ���⣬����Ҫ�����Ȼ��ϵ����NH4Cl������������Һ��V2O5�������ȣ����¶ȡ�������ͼ�Խ�������Ȼ��ϵ��Ϊ_________�������¶ȵķ���Ϊ_________________��
��4������Һ1����Һ2��Ϻ����������������Ԫ�ر���ԭΪ��ͼۣ��䷴Ӧ�����ӷ�Ӧ����ʽΪ___��
��5���������ط�����ã�NH4VO3�ڱ��չ����У����������ļ���ֵ�������꣩���¶ȱ仯��������ͼ��ʾ����NH4VO3�ڷֽ������_________��

A���ȷֽ�ʧȥH2O���ٷֽ�ʧȥNH3 B���ȷֽ�ʧȥNH3���ٷֽ�ʧȥH2O
C��ͬʱ�ֽ�ʧȥH2O��NH3D��ͬʱ�ֽ�ʧȥH2��N2��H2O
��6��ȫ����صĵ������ҺΪVOSO4��Һ����صĹ���ԭ��ΪVO2+ + V2++2H+ ![]() VO2+ +H2O +V3+����س��ʱ�����ĵ缫��ӦʽΪ___________��
VO2+ +H2O +V3+����س��ʱ�����ĵ缫��ӦʽΪ___________��
���𰸡�V2O5+SO32-+4H+=2VO2++SO42-+2H2O SO2+V2O5SO3+V2O4 4VOSO4+O22V2O5+4SO3 4 ����Ӧ��������80���ˮԡ�� 6 VO2++ClO3-+3 H2O=6VO2++Cl-+6 H+ B VO2+ +H2O �Ce- = VO2+ + 2H+
��������
��1������������л�ԭ�ԣ����������£��ܱ���������������������������ӣ����ӷ�Ӧ����ʽΪ��V2O5+SO32-+4H+=2VO2++SO42-+2H2O��
��2������ͼ�е�ת����ϵ��V2O5���뷴Ӧ�����������Ѷ�����������Ϊ��������������ԭΪͼ�в���V2O4������������ԭ��Ӧ��ʵ��д������ƽa����ѧ����ʽSO2+V2O5SO3+V2O4+V2O4��C��VOSO4ת��ΪSO3���˹�����Ҫ�������ɴ���V2O5����Ҫ��������ɣ��˹����е�������Ϊ���������ݻ��ϼ۵ı仯д��C����ѧ����ʽ 4VOSO4+O22V2O5+4SO3��
��3������ͼʾ�������ݣ�80��ʱ���������Ϊ98%���������¶�ʱ������ʷ������ͣ����Ȼ��ϵ��֪���Ȼ��ϵ��Խ�������Խ��ϵ��Ϊ5��Ϊ4����Щ�����Ȼ��ϵ��Խ����Ҫ���Ȼ��Խ�࣬�Ӿ��ýǶȷ��������ʣ����Լ�NH4Cl��ϵ�����Ϊ4�������¶ȵķ���Ϊ����Ӧ��������80���ˮԡ�У�
��4������Һ1����Һ2��Ϻ����������������Ԫ�ر���ԭΪ��ͼ�-1�ۣ��䷴Ӧ�����ӷ�Ӧ����ʽΪ6 VO2++ClO3-+3 H2O=6VO2++Cl-+6 H+��
��5������NH4VO3�ڱ��ձ仯��ͼ���֪��
2NH4VO3�TV2O5+2NH3��+H2O
234g 34g 18g
210��ʱ��������������ֵΪ1-85.47%=14.54%��380��ʱ��������������ֵΪ85.47-77.78%=7.69%��
���ݷ���ʽ֪������ˮʱ�����������ٷ���С�����ɰ���ʱ������210��ʱ���ٵ��ǰ�����380��ʱ���ٵ���ˮ����÷�Ӧ��������ʧȥ������ʧȥˮ����ѡB��
��6��ȫ����صĵ������ҺΪVOSO4��Һ����صĹ���ԭ��ΪVO2+ + V2++2H+ ![]() VO2+ +H2O +V3+����س��ʱ����VO2+ʧ��������VO2+����缫��ӦʽΪVO2+ +H2O �Ce- = VO2+ + 2H+��
VO2+ +H2O +V3+����س��ʱ����VO2+ʧ��������VO2+����缫��ӦʽΪVO2+ +H2O �Ce- = VO2+ + 2H+��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�



