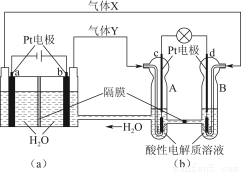
МвДҝДЪИЭ
јЧҙјЦКЧУҪ»»»ДӨИјБПөзіШЦРҪ«јЧҙјХфЖшЧӘ»ҜОӘЗвЖшөДБҪЦЦ·ҙУҰФӯАнКЗ
ўЩCH3OH(g)Ј«H2O(g)=CO2(g)Ј«3H2(g)ЎЎҰӨH1ЈҪЈ«49.0 kJ/mol
ўЪCH3OH(g)Ј« O2(g)=CO2(g)Ј«2H2(g)ЎЎҰӨH2ЈҪЈӯ192.9 kJ/mol
O2(g)=CO2(g)Ј«2H2(g)ЎЎҰӨH2ЈҪЈӯ192.9 kJ/mol
ПВБРЛө·ЁХэИ·өДКЗ(ЎЎЎЎ)
AЈ®1 mol CH3OHНкИ«ИјЙХ·ЕіцИИБҝ192.9 kJ
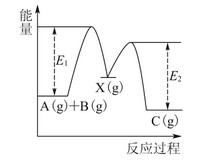
BЈ®ўЪЦРөДДЬБҝұд»ҜИзНјЛщКҫЈ¬ФтQЈҪE3ЈӯE1
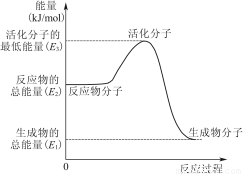
CЈ®H2ИјЙХДЬ·ЕіцҙуБҝөДИИЈ¬№КCH3OHЧӘұдіЙH2өД№эіМұШРлОьКХИИБҝ
DЈ®ёщҫЭўЪНЖЦӘЈәФЪ25 ЎжЈ¬101 kPaКұЈ¬1 mol CH3OH(g)НкИ«ИјЙХЙъіЙCO2әНH2O·ЕіцөДИИБҝУҰҙуУЪ192.9 kJ
D
ЎҫҪвОцЎҝ·ҙУҰўЪІ»КЗјЧҙјөДНкИ«ИјЙХЈ¬AПоҙнОуЈ»ўЪЦРөДДЬБҝұд»ҜУҰОӘНјЦРE2ЈӯE1Ј¬BПоҙнОуЈ»УЙ·ҙУҰўЪЈ¬CH3OHЧӘұдіЙH2ОӘ·ЕИИ№эіМЈ¬CПоҙнОуЈ»H2ИјЙХЙъіЙH2OКұ»№ТӘ·ЕіцИИБҝЈ¬№КDПоХэИ·

Б·П°ІбПөБРҙр°ё
 ҪЧМЭјЖЛгПөБРҙр°ё
ҪЧМЭјЖЛгПөБРҙр°ё
Па№ШМвДҝ