��Ŀ����
19����֪����CO��g��+H2O��g���TCO2��g��+H2��g����H1����CH4��g��+H2O��g���TCO��g��+3H2��g����H2�����ƶ���ȷ���ǣ�������
| A�� | ��CO��ȼ����Ϊ��H3����H2��ȼ����Ϊ��H3-��H1 | |
| B�� | ��ӦCH4��g��+CO2��g���T2CO��g��+2H2��g���ġ�H=��H${\;}_{{\;}_{2}}$-��H1 | |
| C�� | ����Ӧ�ڵķ�Ӧ�����������������������������H2��0 | |
| D�� | �������ʵ�����CO��H2��ȫȼ��������̬����ʱǰ�߷��ȸ��࣬���H1��0 |
���� A������ȼ����ָ�����ȶ��Ļ����ˮӦΪҺ̬�жϣ�
B�����ݸ�˹���ɵâ�-�٣�CH4��g��+CO2��g���T2CO��g��+2H2��g���ġ�H=��H${\;}_{{\;}_{2}}$-��H1��
C������Ӧ�ڵķ�Ӧ����������������������������÷�ӦΪ���ȷ�Ӧ�жϣ�
D������CO��g��+$\frac{1}{2}$02��g���TCO2��g����H3��H2��g��+$\frac{1}{2}$02��g���TH2O��g����H4���������ʵ�����CO��H2��ȫȼ��������̬����ʱǰ�߷��ȸ��࣬���ԡ�H3����H4���ݴ˽�ϸ�˹���ɼ����жϣ�
��� �⣺A����CO��ȼ����Ϊ��H3�����CO��g��+$\frac{1}{2}$02��g���TCO2��g����H3�����ݸ�˹���ɵâ�-�٣�H2��g��+$\frac{1}{2}$02��g���TH2O��g����H3-��H1��ȼ����ָ�����ȶ��Ļ����ˮӦΪҺ̬����A����
B�����ݸ�˹���ɵâ�-�٣�CH4��g��+CO2��g���T2CO��g��+2H2��g���ġ�H=��H${\;}_{{\;}_{2}}$-��H1����B��ȷ��
C������Ӧ�ڵķ�Ӧ����������������������������÷�ӦΪ���ȷ�Ӧ�����ԡ�H2��0����C����
D������CO��g��+$\frac{1}{2}$02��g���TCO2��g����H3��H2��g��+$\frac{1}{2}$02��g���TH2O��g����H4���������ʵ�����CO��H2��ȫȼ��������̬����ʱǰ�߷��ȸ��࣬���ԡ�H3����H4���ָ��ݸ�˹���ɢ�CO��g��+H2O��g���TCO2��g��+H2��g����H1=��H3-��H4��0����D����
��ѡB��
���� ���⿼�黯ѧ��Ӧ�������������ڸ�˹���ɵ����ã�ע����ո�˹���ɵ�ԭ���Լ����㷽�����ѶȲ���

 ��У��������ĩ��̾�ϵ�д�
��У��������ĩ��̾�ϵ�д�
��1��������[Cu��En��2]2+���������ӻ�̬��Χ�����Ų�ʽΪ3d9���Ҷ��������е�ԭ�ӹ�����ӻ�����Ϊsp3��������[Cu��En��2]2+�е���λ��Ϊ4��
��2���Ҷ��������װ�[N��CH3��3]�����ڰ��࣬���Ҷ��������װ��ķе�ߵĶ࣬ԭ�����Ҷ�������֮������γ���������װ�����֮�䲻���γ������
��3���Ƚϱ����д�С���á����ڡ���С�ڡ������ڡ���գ�
| ��һ������ | ���Ӱ뾶 | �۵� | ���� |
| P��S | H-��Li+ | KCl��Si | HClO3��HClO4 |
A��ͼ��aλ����Clԭ��Ϊsp�ӻ������
B��M�Ļ�ѧʽȷ����KCuCl3����N�Ļ�ѧʽΪK2CuCl3��
C��������[CuCl4]2-��������[AlCl4]-�ռ�ṹ���ƣ�
����ͭ�����백ˮ����������ⶼ���ܷ�Ӧ�������백ˮ��������Ļ����Һ��Ӧ����Ӧ�����ӷ�Ӧ����ʽΪCu+H2O2 +4NH3�TCu��NH3��42++2OH-��
��5�����������ʵľ����ڲ�ͬ�¶��������ֶѻ���ʽ�������ֱ���ͼ3��ʾ������֪�������������������ľ����ѻ��Ŀռ������ʷֱ�Ϊ68%��74%������ʽ�������־�����ܶ�֮��[�ѣ�a�����ѣ�b��]Ϊ3$\sqrt{3}$��4$\sqrt{2}$������ɼĹ�ϵʽ�������������ֻ��д������÷֣���
| A�� | �٢ڢ� | B�� | �ڢ٢� | C�� | �٢ۢ� | D�� | �ۢ٢� |
| A�� | ľ̿ | B�� | ��˿ | C�� | �ƾ� | D�� | ���� |
| �� �� | �� �� | �� �� | |
| A | ��ij��Һ�еμ�BaCl2��Һ��ϡHNO3 | ������ɫ���� | ��Һ��һ������SO42- |
| B | ���ۺ�ϡ�����Ϲ��Ⱥ��ټ���������������ͭ����Һ | ������ɫ���� | ����ˮ������������� |
| C | ��ʢ��0.5mol•L-1Fe��NO3��2��Һ���Թ��м���0.5mol•L-3H2SO4��Һ | ���Թܿڴ��ֺ���ɫ���� | ��Һ��NO3-��Fe2+��ԭΪNO2 |
| D | ��ij��ɫ��Һ�еμ���ˮ��CCl4�������� | �²���Һ����ɫ | ԭ��Һ����I- |
| A�� | A | B�� | B | C�� | C | D�� | D |
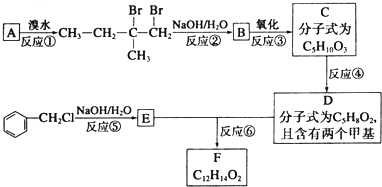
 ��
�� ��
��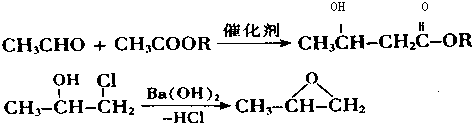
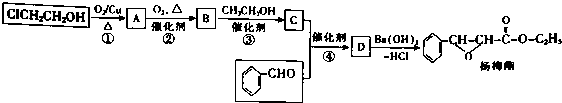
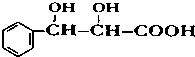 �� �����йظ����ʵ������У���ȷ����CD��
�� �����йظ����ʵ������У���ȷ����CD��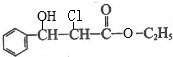 ��
�� �ȣ�
�ȣ�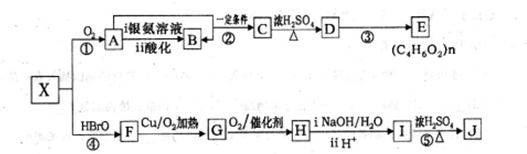

 ����
���� ��
��