��Ŀ����
4��һ�������KMnO4��Һǡ��������һ��������KHC2O4•H2C2O4•2H2O������0.1000mol•L-1��NaOH��Һ�к���ͬ������KHC2O4•H2C2O4•2H2O������NaOH��Һ�����ǡ��ΪKMnO4�ܵ�3������KMnO4��Һ��Ũ�ȣ�mol•L-1��Ϊ����������ʾ��H2C2O4�Ƕ�Ԫ���ᣮ| A�� | 0.008889 | B�� | 0.08000 | C�� | 0.1200 | D�� | 0.2400 |
���� ����Ӧ��KHC2O4•H2C2O4•2H2O�����ʵ�����ͬ���������ʵ���Ϊ1mol��H2C2O4�Ƕ�Ԫ���ᣬ����1molKHC2O4•H2C2O4•2H2O�����к���������3mol������V=$\frac{n}{c}$��������������Һ�����������������������Һ��������ɷ���ʽ10[KHC2O4•H2C2O4]+8KMnO4+17H2SO4=8MnSO4+9K2SO4+40CO2��+32H2O����1molKHC2O4•H2C2O4��Ӧ��ҪKMnO4�����ʵ�����������c=$\frac{n}{V}$������������Һ��Ũ�ȣ�
��� �⣺����Ӧ��KHC2O4•H2C2O4•2H2O�����ʵ�����ͬ���������ʵ���Ϊ1mol��H2C2O4�Ƕ�Ԫ���ᣬ
����1molKHC2O4•H2C2O4•2H2O�����к���������3mol��ǡ������Ҫ����������Һ�����Ϊ��$\frac{3mol}{0.1mol/L}$=30L��
���Ը��������Һ�����Ϊ��30L��$\frac{1}{3}$=10L���ɷ���ʽ10[KHC2O4•H2C2O4]+8KMnO4+17H2SO4=8MnSO4+9K2SO4+40CO2��+32H2O
��֪1molKHC2O4•H2C2O4��Ӧ��ҪKMnO4�����ʵ���Ϊ��$\frac{8}{10}$��1mol=0.8mol��
���Ը��������Һ��Ũ��Ϊ��$\frac{0.8mol}{10L}$=0.08mol/L��
��ѡB��
���� ���⿼�����ʵ���Ũ�ȼ��㡢���ݷ���ʽ�ļ��㣬��Ŀ�Ѷ��еȣ��ж�ǡ�÷����кͷ�Ӧ����������KHC2O4•H2C2O4•2H2O��ϵ�ǹؼ���ע����跨�����ã�����������ѧ������֪ʶ��������

| A�� | N2��H2�ڵ�ȼ����������ºϳɰ� | |
| B�� | �����ȶ��Ա������� | |
| C�� | ����������ˮ����ˮ�ʼ��ԣ���Ҫ�� NH4+��OH- | |
| D�� | �ɰ���ȡ����淋Ĺ����У������ֳ���ԭ�Ժͼ��� |
| A�� | �ɼ�����ȴ�����4�֣�����֪������ȴ�����6�� | |
| B�� | ��ϩ�ͱ���������ʹ��ˮ��ɫ�����ܼ�����ϩ�ͱ����� | |
| C�� | ���ۡ���֬��������һ�������¶��ܷ���ˮ�� | |
| D�� | ��ϩ�;���ϩ����ʹ������Ȼ�̼��Һ��ɫ |
| A�� | ���ӻ�������һ�����н��������� | |
| B�� | ���ӻ�������ֻ�������Ӽ� | |
| C�� | ��������У�������һ���������ӻ����� | |
| D�� | ���Ӽ�һ�������ڻ������� |
| A�� | ����������Һ�У�Cu2+��Fe3+��NO3-��Cl- | |
| B�� | ʹpH��ֽ��������Һ�У�NH4+��Na+��SO42-��Cl- | |
| C�� | ���д���ClO-����Һ�У�K+��OH-��I-��SO32- | |
| D�� | c��Fe3+��=0.1mol•L-1����Һ�У�K+��ClO-��SO42-��SCN- |
| A�� | ������SO2ͨ�뺬Fe2+��Cl-��Ba2+��Al3+����Һ�У������������ܴ������� | |
| B�� | ��NaClO��Һ��ͨ������������̼�����ӷ���ʽ��2ClO-+CO2+H2O�T2HClO+CO32- | |
| C�� | ����������������HBr��Һ��Ӧ�����ӷ���ʽ��Fe��OH��2+3H+�TFe2++3H2O | |
| D�� | ��100mL1mol•L-1��FeCl3��Һ������NaS�����ַ�Ӧ�����ɳ���10.4g |
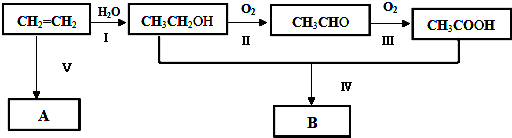
 �����Ƶ�A�Ĺ���ʽ���á�*���������̼ԭ�ӣ�����ϵͳ������������
�����Ƶ�A�Ĺ���ʽ���á�*���������̼ԭ�ӣ�����ϵͳ������������