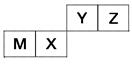
��Ŀ����
X��Y��Z��W��Ԫ�����ڱ�ǰ�������г�����Ԫ�أ��������Ϣ���±���
| Ԫ�� | �����Ϣ |
| X | X�Ļ�̬ԭ�Ӻ���������ܼ��ϵ�������� |
| Y | Y��Xͬ���ڣ�Y��̬ԭ��p�ܼ��ijɶԵ�������δ�ɶԵ�������� |
| Z | Z�ĵ�����һ������ɫ���ý������ڿ�����ȼ�պ����ɵ���ɫ�Ĺ��� |
| W | ��W2+����Һ�еμ�ǿ����ɫ���������ڿ�����Ѹ�ٱ�ɻ���ɫ������ɺ��ɫ |
��1��Wλ�����ڱ��ĵ�______����_____�壬���̬ԭ���������________�����ӡ�
��2��X������Ȼ�����ӳ�____________�ͽṹ�� Z�ڿ�����ȼ�����ɵĵ���ɫ�Ĺ����к��еĻ�ѧ������____________________��
��3����������ZͶ�뺬WCl3����Һ�У�������Ӧ�����ӷ���ʽΪ____________________��
��4����ҵ��ұ��W�����漰���������Ȼ�ѧ����ʽ��
3W2Y3(s)��XY(g) =2W3Y4(s)��XY2(g) ��H=��15.73kJ/mol
W3Y4(s)��XY (g) =" 3WY" (s)��XY 2(g) ��H= +640.4kJ/mol
��ӦW2Y3 (s)��XY (g) =" 2" WY (s)��XY 2(g) �ġ�H= _________________ ��
��1���ġ� ���� 2
��2���������塢 ���Ӽ� ���Ǽ��ԣ����ۼ�
��3��6Na+6H2O+2Fe3+=6Na++3H2��+2Fe(OH)3��
��4����H= +421.69kJ/mol.
�������������������Ŀ�ṩ����Ϣ��֪��XΪC��YΪO��ZΪNa;W��Fe����1��26��Ԫ��Fe��Ԫ�����ڱ���λ�ڵ������ڵڢ��塣��̬ԭ���������2�����ӡ���2��C������Ȼ������CCl4����������ṹ��Na�ڿ�����ȼ�ղ���Na2O2. �����ӻ�����Na2O2�к���Na��O֮������Ӽ���O��O֮��ķǼ��Թ��ۼ�����3��NaͶ�뵽FeCl3��Һ��ʱ�����ȷ�����Ӧ��2Na+2H2O=2NaOH+H2����Ȼ������FeCl3+3NaOH=Fe(OH)3��+3NaCl.�ܷ���ʽΪ6Na+6H2O+2FeCl3=6NaCl+3H2��+2Fe(OH)3��.���ӷ���ʽΪ��6Na+6H2O+2Fe3+=6Na++3H2��+2Fe(OH)3�� ������+�ڡ�2����3�����ɵ�Fe2O3 (s)��CO(g) =" 2" FeO (s)��CO2(g) ��H= +421.69kJ/mol.
���㣺����Ԫ�ص�λ�á�ԭ�ӽṹ�����ӵĿռ乹�͡���ѧ�������͡����ӷ���ʽ���Ȼ�ѧ����ʽ����д��

�±���Ԫ�����ڱ���һ����, ��Ա��еĢ١�����Ԫ��,��д���пհ�:
| ���� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0�� |
| 1 | �� | | | |||||
| 2 | | | | �� | �� | �� | | |
| 3 | �� | | �� | | �� | | �� | �� |
| 4 | �� | | | | | | | |
��1��д������Ԫ����ɵķ����е�������Ϊ10�ķ��ӣ��������֣��� ��
��2���Ƚϣ��ڡ��ۡ��ܡ��ݵĵ�һ�����ܣ� �� �� �� ��дԪ�ط��ţ���
��3��������������ˮ�����У�������ǿ�Ļ�����ķ���ʽ�� ��������ǿ�Ļ�����ĵ���ʽ��:
��4���������Ե������������ (�ѧʽ)��д����������������Һ��Ӧ�����ӷ���ʽ�� ��
��5���±�Ϊԭ��������������Ķ�����Ԫ��A��E�ĵ�һ��������������ݡ�
| ������I(eV) | A | B | C | D | E |
| I1 | 11��3 | 13��6 | 5��2 | 7��6 | 6��0 |
| I2 | 24��4 | 35��1 | 49��3 | 15��0 | 18��8 |
| I3 | 47��9 | 54��9 | 71��6 | 80��1 | 28��4 |
| I4 | 64��5 | 77��4 | 98��9 | 109��2 | 112��0 |
| I5 | 392��1 | 113��9 | 138��3 | 141��3 | 153��7 |
�Իش𣺱��п���Ϊ�ǽ���Ԫ�ص��� ������ĸ������D��EΪͬ��������Ԫ�أ�����D��E�ĵ�һ�������Դ���ԭ���� ������ ��
�±���Ԫ�����ڱ���һ���֣���Ա��еĢ١�����Ԫ�أ���д���пհף�
| ���� ���ڡ��� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0�� |
| 2 | | | | �� | �� | �� | | |
| 3 | �� | | �� | | | �� | �� | �� |
| 4 | �� | | | | | | | |
��________����________����________����________��
(2)д�����з�Ӧ�Ļ�ѧ����ʽ��
�ߵ��ʸ��ܵ�����������Һ��Ӧ��______________________________
�ݵ���������ܵ�����������Һ��Ӧ��________________________________
�ĵ����ڢ۵ĵ�����ȼ�գ�_____________________________________��
������ѧ��ѧ����Ԫ��ԭ�ӽṹ���������±���ʾ��
| ��� | Ԫ�� | �ṹ������ |
| �� | A | A�����������еij������������������Ȼ����Է����������35.5 |
| �� | B | Bԭ���������������ڲ����������1/5 |
| �� | C | C�dz������ʵ���ҪԪ�أ����ʳ����³���̬ |
| �� | D | D���ʱ���Ϊ����Ϣ�����Ĵ��������dz��õİ뵼����� |
| �� | E | ͨ������£�Eû�������ϼۣ�A��B��C��D��F������E�γɻ����� |
| �� | F | F�����ڱ��п������ڢ�A�壬Ҳ����������ڢ�A�� |
��1��AԪ�������ڱ��е�λ��Ϊ_______________________________________________��
��2��B��C�γɵĻ�����Ļ�ѧʽΪ_______________________________________��������________(����ӡ����ۡ�)�����
��3����F��E�����γ�ԭ�Ӹ����ȷֱ�Ϊ2��1��1��1�����ֻ�����X��Y������X��Y��ˮ��Һ��ʵ�鷽����__________________________________________��
��F��C��ɵ����ֻ�����M��N�����ĵ������ֱ���X��Y��ȣ���M��ˮ��Һ��________________�ԣ�N�ĽṹʽΪ______________________________��
��4��C��E���ǽϻ��õķǽ���Ԫ�أ��û�ѧ����ʽ���������ֵ��ʵ�������ǿ����________________________________________________________��
��5��������ΪB��D�ĵ����õ������Ӻ����NaOH��Һ�п����γ�ԭ��أ�����Ϊ�Ƿ���ԣ������ԣ���д�������ĵ缫����ʽ(����Ϊ���пɲ�д)��______________________________________________________________��