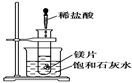
��Ŀ����
�ྦྷ�裨�赥�ʵ�һ�֣�����Ϊ�����Ӵ��õĻ�ʯ�����Ʊ��и�������SiCl4Ϊ������������Ⱦ�ܴ�����ˮǿ��ˮ�⣬�ų��������ȡ��о���Ա����SiCl4ˮ�����ɵ�����ͱ���ۣ���Ҫ�ɷ�ΪBaCO3���Һ�������þ�����ӣ��Ʊ�BaCl2��2H2O�������������£�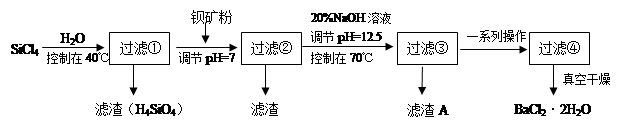
��֪��
�ٳ�����Fe3+��Mg2+��ȫ������pH�ֱ���3.4��12.4
��BaCO3����Է���������197��BaCl2��2H2O����Է���������244
�ش��������⣺
��1��SiCl4����ˮ�ⷴӦ�Ļ�ѧ����ʽΪ_______________________________________
��2����H2��ԭSiCl4��������ȡ���ȺܸߵĹ裬����Ӧ����1mol����ת��ʱ����59KJ��������÷� Ӧ���Ȼ�ѧ����ʽΪ_____________________________________________
��3���ӱ���۲�����pH=7��Ŀ���Ǣ� ����
��4�����ˢں����Һ��Fe3+Ũ��Ϊ ����Һ�¶�25�棬Ksp[Fe(OH)3]=2.2��10-38��
��5����������A�����ӷ���ʽ__________________________________________
��6����ʽ�����10�ֺ�78.8% BaCO3�ı�������������������BaCl2��2H2O������Ϊ���ٶ֣�
(14�֣���2��6�ʸ�3�֣�������2��)��1��SiCl4+4H2O��H4SiO4��+4HCl
��2��SiCl4(s)+2H2(g)��Si(s)+4HCl(s) ��H��+236kJ/mol
��3��ʹBaCO3ת��ΪBaCl2��ʹFe3+��ȫ���� ��4��2.2��10-17mo/L
��5��Mg2����2OH����Mg(OH)2�� ��6��= ��244��9.76t
��244��9.76t
���������������1���Ȼ���ˮ������ԭ������Ȼ��⣬ˮ�ⷽ��ʽΪSiCl4+4H2O��H4SiO4��+4HCl��
��2���ڷ�Ӧ�й�Ԫ�صĻ��ϼ۴ӣ�4�۽��͵�0�ۣ��õ�4�����ӡ�����Ӧ����1mol����ת��ʱ����59KJ������������1mol�Ȼ��������յ�������59kJ��4��236kJ����˸÷�Ӧ���Ȼ�ѧ����ʽΪSiCl4(s)+2H2(g)��Si(s)+4HCl(s) ��H��+236kJ/mol��
��3��pH��3.4ʱ��������������ȫ���ɳ����������̼�ᱵ��Ӧ�����Ȼ����Ͷ�����̼��ˮ�����Լӱ���۲�����pH��7��������ʹBaCO3ת��ΪBaCl2��ͬʱʹFe3+��ȫ������
��4����Һ��pH��7������Һ�� c(OH��)��1��10��7mo/L���������������ܶȻ�������֪����Һ��Fe3+Ũ�ȣ�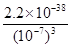 ��2.2��10-17mo/L��
��2.2��10-17mo/L��
��5��������Һ�л�����þ���ӣ�������Ҫ������Һ��pH��12.5��ʹþ������ȫ��������������þ����Ӧ�����ӷ���ʽ��Mg2����2OH����Mg(OH)2��
��6�����ݱ�ԭ���غ��֪��10�ֺ�78.8% BaCO3�ı�������������������BaCl2��2H2O������Ϊ ��244��9.76t��
��244��9.76t��
���㣺�����Ʊ�ʵ�鷽������ƣ��Ȼ�ѧ����ʽ����д�����ܵ���ʵ��ܽ�ƽ�⼰����ת���ļ��㣻�����Ʊ��ļ����

������������Ȼ����Ϊԭ�Ϻϳɼ״������ⱻһһ���ˣ�����شٽ��˼״���ѧ�ķ�չ��
��1����̿��ˮ�����ķ�Ӧ���ƣ�����Ȼ��Ϊԭ��Ҳ�����Ƶ�CO��H2���÷�Ӧ�Ļ�ѧ����ʽΪ_________��
��2���ϳɼ״���һ�ַ�������CO��H2Ϊԭ�ϣ��������仯��ͼ��ʾ��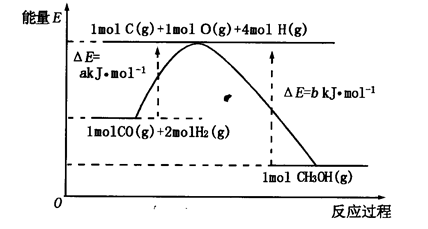
��ͼ��֪���ϳɼ״����Ȼ�ѧ����ʽΪ________________________________________��
��3����CO2Ϊԭ��Ҳ���Ժϳɼ״����䷴Ӧԭ��Ϊ��CO2(g)+3H2(g) CH3OH(g)+H2O(g)
CH3OH(g)+H2O(g)
����lL���ܱ������У�����1molCO2��3molH2����500���·�����Ӧ�����CO2(g)��CH3OH(g)��Ũ����ʱ�ʱ仯��ͼ��ʾ��
������˵����ȷ����_________________(����ĸ)��
| A��3minʱ��Ӧ�ﵽƽ�� |
| B��0��10minʱ��H2��ʾ�ķ�Ӧ����Ϊ0��225mol��-1��min-1 |
| C��CO2��ƽ��ת����Ϊ25�� |
D�����¶�ʱ��ѧƽ�ⳣ��Ϊ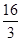 ��mol/L����2 ��mol/L����2 |
| ���� | ����1 | ����2 | ����3 |
| ��Ӧ��Ͷ������ʼ̬�� | 1molCO2��3molH2 | 0.5molCO2��1.5molH2 | 1molCH3OH��1molH2O |
| CH3OH��ƽ��Ũ��/mol?L-1 | c1 | c2 | c3 |
| ƽ��ʱ��ϵѹǿ/Pa | p1 | p2 | p3 |
�����и����Ĵ�С��ϵΪc1___________c3��p2_________p3(����ڡ��������ڡ���С�ڡ�)��
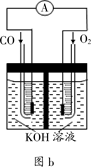
��4�����������״�ȼ�ϵ�ؼ���������µ�ͻ�ƣ���ͼ��ʾΪ�״�ȼ�ϵ�ص�װ��ʾ��ͼ����ع���ʱ���ֱ��b��c����CH3OH��O2���ش��������⣺

�ٴ�d���ų���������___________����Һ�е���������缫__________(�M����N��)��
�ڵ缫M�Ϸ����ĵ缫��ӦʽΪ__________________________��
Ŀǰ��ҵ�ϳɰ���ԭ���ǣ�N2(g)+3H2(g) 2NH3(g) ��H=��93.0kJ /mol�����ݱ�����һ�������£�2N2(g)+6H2O(l)
2NH3(g) ��H=��93.0kJ /mol�����ݱ�����һ�������£�2N2(g)+6H2O(l) 4NH3(g)+3O2(g) ��H=" +1530.0kJ" /mol��
4NH3(g)+3O2(g) ��H=" +1530.0kJ" /mol��
��1��������ȼ���ȡ�H=_______________kJ/mol��
��2���ں��º�ѹװ���н��й�ҵ�ϳɰ���Ӧ������˵����ȷ���� ��
| A������������ٱ仯������ƽ�� |
| B�������ܶȲ��ٱ仯����δƽ�� |
| C��ƽ�����װ����ͨ��һ����Ar��ѹǿ���䣬ƽ�ⲻ�ƶ� |
| D��ƽ���ѹ��װ�ã����ɸ���NH3 |
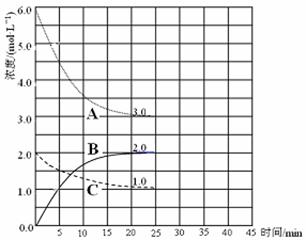
�� ��ʾN2Ũ�ȱ仯�������� ��
�� ǰ25 min �ڣ���H2Ũ�ȱ仯��ʾ�Ļ�ѧ��Ӧ������ ��
�� ��25 minĩ�պ�ƽ�⣬��ƽ�ⳣ��K = ��
��4���ڵ�25 min ĩ�����������������䣬�����¶ȣ��ڵ�35 minĩ�ٴ�ƽ�⡣ƽ���ƶ�������H2Ũ�ȱ仯��1.5 mol��L��1����ͼ�л�����25 min �� 40 min NH3Ũ�ȱ仯���ߡ�
��5����֪�����£�NH4+ ��ˮ�ⳣ��Ϊ1.0��10��9����0.1mol/L NH4Cl��ҺpH= ��������NH4+ˮ���NH4+Ũ�ȵ�Ӱ�죩
��һ�������£���ӦN2+3H2 2NH3����2L�ܱ������н��У�5min�ڰ�������������1.7g����Ӧ����Ϊ��
2NH3����2L�ܱ������н��У�5min�ڰ�������������1.7g����Ӧ����Ϊ��
| A��v��NH3��="0.1" mol����L��min�� | B��v��N2��="0.02" mol����L��min�� |
| C��v��H2��="0.015" mol����L��min�� | D��v��NH3��="0.17" mol����L��min�� |
 CO(g) + 3H2(g) ��H>0
CO(g) + 3H2(g) ��H>0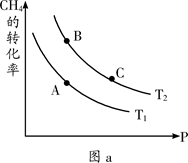
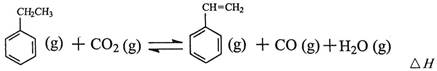
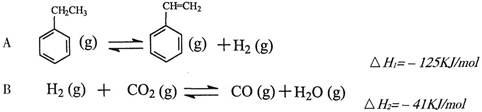
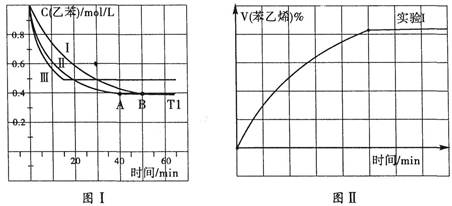
 2CO2(g)+S(l) ��H
2CO2(g)+S(l) ��H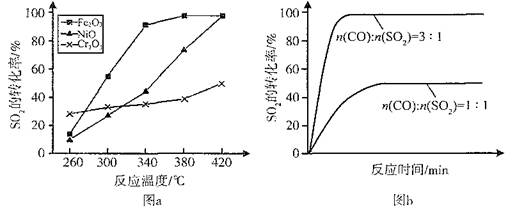
 HSO3����ƽ�ⳣ��K="8.0" �� 106 L?mol��1������ʱSO2��H2SO3��Ũ�Ⱥ��Բ��ƣ�
HSO3����ƽ�ⳣ��K="8.0" �� 106 L?mol��1������ʱSO2��H2SO3��Ũ�Ⱥ��Բ��ƣ�