��Ŀ����
����Ŀ������Ͻ����Բ�Ϊ�������Ԫ�Ͻ��ڸ����£�������������ܣ��������Ϊ��������������Ͻ���Լ�ǿ�����ȶ��Խϸߣ��ͻ�ѧ��ʴ�Ժܺã���Ҫ���ں��캽���DZ������ӱ��ſعܵȡ�
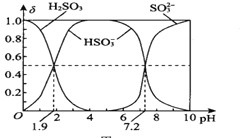
��1����̬��ԭ�ӵļ۵����Ų�ͼΪ______��
��2�����ȶ���ऺϲ�����Pt2+��Cl-����ऽ���γɵIJ�������˳ʽ�ͷ�ʽ����ͬ���칹�壨��ͼ������ѧ�о�������˳ʽ���Ӿ��п������ԡ�

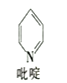
����ष����Ǵ����ƽ�����壬��ṹ��ʽ��ͼ��ʾ��ÿ�������к��еĦҼ���ĿΪ______�����ȶ���ऺϲ�������������C��N��Cl����Ԫ�صĵ�һ�������ɴ�С��˳����______��
�ڶ��ȶ���ऺϲ��д��ڵ�������������______������ĸ����
a�����»���b�����c��������d�Ǽ��Լ�
�۷�ʽ���ȶ���ऺϲ�������______������Է��ӡ��Ǽ��Է��ӡ�����
��3��ij�о�С�齫ƽ���͵IJ��������ӽ��в�״������ʹÿ�������еIJ�ԭ����ijһ���������г��У������ܵ���ġ����ӽ���������ṹ��ͼ��ʾ��

�١����ӽ��������Ե��磬����Ϊ______���������еĽ���ԭ����������
�ڡ����ӽ������У���ԭ���Ƿ���sp3�ķ�ʽ�ӻ���______����ǡ�������������______��
��4���������Ͽ�ѧ����ʵ����һ������С�鷢������5K�³��ֳ����Եľ���CoO2���þ�����в�״�ṹ����ͼ��ʾ��С���ʾCoԭ�ӣ������ʾOԭ�ӣ���ͼ���ô��������ظ��ṹ��Ԫʾ��ͼ��������CoO2�Ļ�ѧ��ɵ���______������ĸ����

��5��������Ʒ���У���ԭ�ӵ���λ��Ϊ12��������������ʽx��y��z���ͶӰͼ��ͼ��ʾ�������������ܶ�Ϊdgm-3��������a=______nm���м���ʽ����

���𰸡�![]() 11 N>Cl>C ad �Ǽ��Է��� ���� �� ����ԭ�ӹ��Ϊsp3�ӻ�����÷��ӽṹΪ�����壬��ƽ��ṹ B
11 N>Cl>C ad �Ǽ��Է��� ���� �� ����ԭ�ӹ��Ϊsp3�ӻ�����÷��ӽṹΪ�����壬��ƽ��ṹ B ![]() ��
��![]()
��������
��1�����ɽ����ļ۵��Ӱ���3d�ܼ���4s�ܼ��ĵ��ӣ�
��2����������ԭ�Ӽ�ĵ�һ�����ۼ�Ϊ������ͼ�и�����Ϊ���ȶ���ऺϲ�������ष��ӣ�![]() ��������ʽΪC5H5N��C-H����C-N��C-C��һ��Ϊ����������11������һ������ͬ�����У�����������ƣ����ڳ���������״̬ʱ�������ĵ����ܣ��ɴ�С��˳��Ϊ��N>Cl>C��
��������ʽΪC5H5N��C-H����C-N��C-C��һ��Ϊ����������11������һ������ͬ�����У�����������ƣ����ڳ���������״̬ʱ�������ĵ����ܣ��ɴ�С��˳��Ϊ��N>Cl>C��
�ڶ��ȶ���ऺϲ��У�C-C����ڷǼ��Լ���Pt-N�������λ�������Ӽ���ڷ��»�����
�۸���ͼ���֪����ʽ���ȶ���ऺϲ����ӵĽṹΪ��ȫ�Գƣ�������������غϣ������ǷǼ��Է��ӣ�
��3������֪��ÿ�������еIJ�ԭ����ijһ���������г��У������ܵ���������ӽ���������Pt�����ļ۵��ӿ��ڽ���Pt�䴫�ݶ����磻
�������ӽ������У���ԭ�ӵ���λ��Ϊ4������ԭ����sp3�ķ�ʽ�ӻ�����Ϊ�����幹�ͣ���ͼ�и�������Ϣ��֪�������ӽ�����Ϊƽ�湹�ͣ�
��4�����ݾ���Ļ�ѧʽ��֪��Co��O=1��2��A.Coԭ����Ϊ1��Oԭ��Ϊ4��![]() =2�����ϱ�����B. Coԭ����Ϊ1��Oԭ��Ϊ4��
=2�����ϱ�����B. Coԭ����Ϊ1��Oԭ��Ϊ4��![]() =1�������ϱ�����C. Coԭ����Ϊ1+4��
=1�������ϱ�����C. Coԭ����Ϊ1+4��![]() =2��Oԭ��Ϊ4�����ϱ�����D. Coԭ����Ϊ4��
=2��Oԭ��Ϊ4�����ϱ�����D. Coԭ����Ϊ4��![]() =1��Oԭ��Ϊ4��
=1��Oԭ��Ϊ4��![]() =2�����ϱ�������ΪB��
=2�����ϱ�������ΪB��
��5�������������У���ԭ�ӵ���λ��Ϊ12��Ϊ���ܶѻ�������ͶӰͼ��֪������Ϊ�����������ܶѻ���һ�������к��еIJ�ԭ�Ӹ���Ϊ��8��![]() +6��
+6��![]() =4��������������ⳤΪa����=
=4��������������ⳤΪa����=![]() =
=![]() ��
��
��1�����ɽ����ļ۵��Ӱ���3d�ܼ���4s�ܼ��ĵ��ӣ���Ϊ��![]() ��
��
��2����������ԭ�Ӽ�ĵ�һ�����ۼ�Ϊ������ͼ�и�����Ϊ���ȶ���ऺϲ�������ष��ӣ�![]() ��������ʽΪC5H5N��C-H����C-N��C-C��һ��Ϊ����������11������һ������ͬ�����У�����������ƣ����ڳ���������״̬ʱ�������ĵ����ܣ��ɴ�С��˳��Ϊ��N>Cl>C��
��������ʽΪC5H5N��C-H����C-N��C-C��һ��Ϊ����������11������һ������ͬ�����У�����������ƣ����ڳ���������״̬ʱ�������ĵ����ܣ��ɴ�С��˳��Ϊ��N>Cl>C��
�ڶ��ȶ���ऺϲ��У�C-C����ڷǼ��Լ���Pt-N�������λ�������Ӽ���ڷ��»�������Ϊad��
�۸���ͼ���֪����ʽ���ȶ���ऺϲ����ӵĽṹΪ��ȫ�Գƣ�������������غϣ������ǷǼ��Է��ӣ�
��3������֪��ÿ�������еIJ�ԭ����ijһ���������г��У������ܵ���������ӽ���������Pt�����ļ۵��ӿ��ڽ���Pt�䴫�ݶ����磻
�������ӽ������У���ԭ�ӵ���λ��Ϊ4������ԭ����sp3�ķ�ʽ�ӻ�����Ϊ�����幹�ͣ���ͼ�и�������Ϣ��֪�������ӽ�����Ϊƽ�湹�ͣ���Ϊ������ԭ�ӹ��Ϊsp3�ӻ�����÷��ӽṹΪ�����壬��ƽ��ṹ��
��4�����ݾ���Ļ�ѧʽ��֪��Co��O=1��2��A.Coԭ����Ϊ1��Oԭ��Ϊ4��![]() =2�����ϱ�����B. Coԭ����Ϊ1��Oԭ��Ϊ4��
=2�����ϱ�����B. Coԭ����Ϊ1��Oԭ��Ϊ4��![]() =1�������ϱ�����C. Coԭ����Ϊ1+4��
=1�������ϱ�����C. Coԭ����Ϊ1+4��![]() =2��Oԭ��Ϊ4�����ϱ�����D. Coԭ����Ϊ4��
=2��Oԭ��Ϊ4�����ϱ�����D. Coԭ����Ϊ4��![]() =1��Oԭ��Ϊ4��
=1��Oԭ��Ϊ4��![]() =2�����ϱ�������ΪB��
=2�����ϱ�������ΪB��
��5�������������У���ԭ�ӵ���λ��Ϊ12��Ϊ���ܶѻ�������ͶӰͼ��֪������Ϊ�����������ܶѻ���һ�������к��еIJ�ԭ�Ӹ���Ϊ��8��![]() +6��
+6��![]() =4��������������ⳤΪa����=
=4��������������ⳤΪa����=![]() =
=![]() ����a=
����a=![]() ��
��![]() ��
��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ���ᴿ��������(������Ϊ����)����ѡ�����Լ�����������ȷ����( )
ѡ�� | ���� | �����Լ� | ���� |
A | �ƾ�(ˮ) | CaO | ���� |
B | ��������(����) | NaOH��Һ | ��Һ |
C | ����(��ϩ) | ���Ը��������Һ | ϴ�� |
D | ��(��) | KI��Һ | ��Һ |
A.AB.BC.CD.D
����Ŀ����ͼ��ʾװ��(����������)�����Ƚ�����Һ�������У��۲쵽�������Թ���һ��ʱ���ָ�ԭ״���ٽ�����Һ����붡�У����������Թ������������ȷ���ǣ� ��

ѡ�� | �ιܼ� | �ձ��� | �ι��� | �ձ��� |
A | ˫��ˮ | �������� | ˮ | �������� |
B | ϡ���� | þ | ���� | ̼��� |
C | ˮ | �������� | ˮ | ����� |
D | ˮ | ������ | ���� | þ |
A.AB.BC.CD.D