��Ŀ����
�Կ��淴ӦN2��g��+3H2��g�� 2NH3��g������H=-92��4 kJ��mol��1������
2NH3��g������H=-92��4 kJ��mol��1������
����ȷ����
 2NH3��g������H=-92��4 kJ��mol��1������
2NH3��g������H=-92��4 kJ��mol��1����������ȷ����
| A���ﵽ��ѧƽ��ʱ���������¶ȣ�������Ӧ���ʼ�С���淴Ӧ�������� |
| B������λʱ��������x mol N2��ͬʱ����2x mol NH3����Ӧ�ﵽƽ��״̬ |
| C�����ﵽƽ��ʱ�����ų�46��2kJ����������l mo1NH3��g������ |
| D���ﵽƽ������������СΪԭ����һ�룬���´ﵽƽ���c��NH3����Ϊԭƽ��ʱ��2�� |
C
�����¶ȣ����淴Ӧ���ʶ�����A����B��ֻ��˵����ƽ�������淽����У�
���ж�����Ӧ�������淴Ӧ�����Ƿ���ȣ�B�����ų���������92��4��һ�룬
������NH3Ϊ1mol��C��ȷ�����������СΪԭ����һ�룬��ƽ���������ƶ���
��c��NH3������ԭ����2����D������ѡC��
���ж�����Ӧ�������淴Ӧ�����Ƿ���ȣ�B�����ų���������92��4��һ�룬
������NH3Ϊ1mol��C��ȷ�����������СΪԭ����һ�룬��ƽ���������ƶ���
��c��NH3������ԭ����2����D������ѡC��

��ϰ��ϵ�д�
�����Ŀ
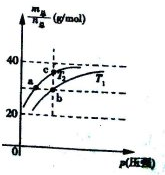
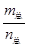 =69g/molʱ����n(NO2)��n(N2O4)=2��1��
=69g/molʱ����n(NO2)��n(N2O4)=2��1��
 N2(g)+CO2(g) ��H="Q" kJ��mol��1��T1��ʱ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
N2(g)+CO2(g) ��H="Q" kJ��mol��1��T1��ʱ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
 2SO3(g)�������������Ҫ��Ӧ֮һ���±���ԭ������
2SO3(g)�������������Ҫ��Ӧ֮һ���±���ԭ������ Ͷ�ϣ���
Ͷ�ϣ���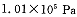 ʱ����ͬ�¶���SO2��ƽ��ת���ʡ�
ʱ����ͬ�¶���SO2��ƽ��ת���ʡ�
 ʱ������10 mol SO2��ԭ����ͨ��һ�ܱ������н��з�Ӧƽ��ʱSO2�����ʵ����� mol��
ʱ������10 mol SO2��ԭ����ͨ��һ�ܱ������н��з�Ӧƽ��ʱSO2�����ʵ����� mol��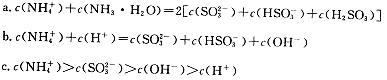

 xC(g)��2D(g)����5 min��ﵽƽ�⣬ƽ��ʱ���D��Ũ��Ϊ0��5 mol��L��1��c(A)��c(B) = 3��5��v(C) = 0��1 mol��L��1��min��1��
xC(g)��2D(g)����5 min��ﵽƽ�⣬ƽ��ʱ���D��Ũ��Ϊ0��5 mol��L��1��c(A)��c(B) = 3��5��v(C) = 0��1 mol��L��1��min��1��
 Ag(s)��Fe3������H��0���ﵽƽ���Ϊʹƽ����ϵ����������������ɲ�ȡ�Ĵ�ʩ�ǣ� ��
Ag(s)��Fe3������H��0���ﵽƽ���Ϊʹƽ����ϵ����������������ɲ�ȡ�Ĵ�ʩ�ǣ� ��