题目内容
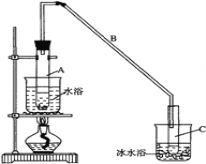
【题目】某化学小组采用如图装置,以环己醇制备环己烯:
密度(g/cm3) | 熔点(℃) | 沸点(℃) | 溶解性 | |
环己醇 | 0.96 | 25 | 161 | 能溶于水 |
环己烯 | 0.81 | -103 | 83 | 难溶于水 |
已知: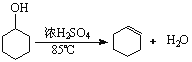
(1)制备粗品
将12.5mL环己醇加入试管A中,再加入1mL浓硫酸,摇匀后放入碎瓷片,缓慢加热至反应完全,
在试管C内得到环己烯粗品。
①试管C置于冰水浴中的目的是 _____________________________________。
②导管B除了导气外还具有的作用是____________,A中碎瓷片的作用是 ___________。
(2)制备精品
①环己烯粗品中含有环己醇和少量酸性杂质等。加入饱和食盐水,振荡、静置、分层,环己烯在______ 层(填“上”或“下”),分液后用___________________(填入编号)洗涤。
A.KMnO4溶液 B.稀H2SO4 C.Na2CO3溶液
②再将环己烯按图装置蒸馏,冷却水从________口进入。蒸馏时要加入生石灰,目的是:_________________________。
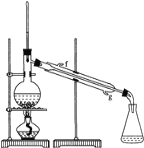
③收集产品时,控制的温度应在________________左右,实验制得的环己烯精品质量低于理论产量,可能的原因是 _______
A.蒸馏时从70℃开始收集产品
B.环己醇实际用量多了
C.制备粗品时环己醇随产品一起蒸出
(3)以下区分环己烯精品和粗品的方法,合理的是_______
A.用酸性高锰酸钾溶液 B.用金属钠 C.测定沸点
【答案】防止环己烯挥发 冷凝 防暴沸 上 C g 除去水分 83C C BC
【解析】
环己醇在浓硫酸存在下加热到85℃生成环己烯,应采用水浴加热,长导管可以起导气和冷凝的作用;分离环己烯中的环己醇和酸性杂质,需要进行分液,然后用碳酸钠溶液洗涤,减少产品中的环己醇和酸性杂质;环己醇能和金属钠反应,但环己烯不能;二者都能和酸性高锰酸钾溶液反应。
(1)①环己烯的沸点较低,为83℃,放在冰水浴中可以防止环己烯挥发;
②长导管有导出气体和冷凝的作用;液体中加入碎瓷片的作用是防止液体加热过程中暴沸;
(2)①环乙烯的密度比水小,在上层,分液后用碳酸钠溶液洗涤,洗去酸性杂质和环己醇等;
②冷凝装置中水流方向是下进上出,即g口进,f口出;生石灰能和水反应,所以蒸馏时加入生石灰能干燥;
③因为环己烯的沸点为83℃,所以控制的温度应在83℃左右;
A.蒸馏时从70℃开始收集产品,则产品的质量高,A错误;
B.环己醇实际用量多了,会使产品的量增加,B错误;
C.制备粗品时环己醇随产品一起蒸出,会使生成的环乙烯量减少,C正确;
故选C;
(3)环己烯粗品中含有少量的环己醇,故可以通过鉴别环己醇的存在,来鉴别环己烯的精品和粗品;
A.环己烯和环己醇都能使酸性高锰酸钾溶液褪色,故不能通过酸性高锰酸钾来鉴别环己烯的精品和粗品,A错误;
B.环己醇能与金属钠反应,但环己烯不能,可以用金属钠来鉴别环己烯的精品和粗品,B正确;
C.测定沸点的方法可以鉴别环己烯的精品和粗品,C正确;
故合理的方法为BC。

 天天向上一本好卷系列答案
天天向上一本好卷系列答案【题目】燃煤的烟气中含有 SO2,为了治理雾霾天气,工厂采用多种方法实现烟气脱硫。
Ⅰ.(1)“湿式吸收法”利用吸收剂与 SO2 发生反应从而脱硫。下列试剂中适合用作该法吸收剂的是_____(填字母序号)。
a. 石灰乳 b.CaCl2溶液
(2)某工厂利用含 SO2 的烟气处理含Cr2O72-的酸性废水,吸收塔中反应后的铬元素以Cr3+形式存在,具体流程如下:

①用 SO2 处理含铬废水时,利用了 SO2 的_____性。
②吸收塔中发生反应的离子方程式为_____。
Ⅱ.石灰-石膏法和烧碱法是常用的烟气脱硫法。石灰-石膏法的吸收反应为Ca(OH)2+SO2= CaSO3↓+H2O。吸收产物亚硫酸钙由管道输送至氧化塔氧化,反应为2CaSO3+O2+4H2O =2CaSO4·2H2O。其流程如图:

烧碱法的吸收反应为2NaOH+SO2=Na2SO3+H2O。该法的特点是氢氧化钠碱性强、吸收快、效率高。其流程如图:

已知:
试剂 | Ca(OH)2 | NaOH |
价格(元/kg) | 0.36 | 2.9 |
吸收 SO2 的成本(元/mol) | 0.027 | 0.232 |
(3)石灰-石膏法和烧碱法相比,石灰-石膏法的优点是_______,缺点是_______。
(4)某学习小组在石灰-石膏法和烧碱法的基础上,设计一个改进的、能实现物料循环的烟气脱硫方案,流程图中的甲、乙、丙各是_____、_____、_____(填化学式)