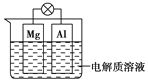
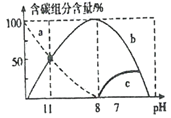
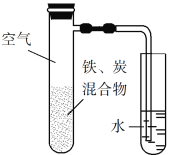
��Ŀ����
����Ŀ������˵����ȷ����
A. �����£�pH=4��CH3COOH��Һ�У���ˮ�����c(H��) = 10-10 mol/L
B. ��pH=2��HCl��Һ��pH=4��H2SO4��Һ�������ϣ�������ҺpH=3
C. 0.1 mol/L��ˮ��0.1 mol/L NH4Cl��Һ�������Ϻ�������ҺpH>7����c(NH4��) < c(NH3��H2O)
D. ��0.1 mol/L��pH=1��NaHA��Һ�м���NaOH��Һ��Ӧ�����ӷ���ʽΪ��HA��+ OH�� = H2O + A2-
���𰸡�A
��������
A. �����£�pH=4��CH3COOH��Һ�У�c(H��) = 10-4mol/L������ˮ�����c(H��) ˮ= c(OH-)ˮ= 10-10 mol/L����A��ȷ��B. ��pH=2��HCl��Һ��c(H��) = 10-2mol/L��pH=4��H2SO4��Һ��c(H��) = 10-4mol/L�����ߵ������ϣ�������Һ��c(H��) = ![]() ��(10-2mol/L +10-4mol/L)��
��(10-2mol/L +10-4mol/L)��![]() ��10-2mol/L��10-3mol/L��pH��3����B����C. 0.1 mol/L��ˮ��0.1 mol/L NH4Cl��Һ�������Ϻ�������ҺpH>7����c(H+)��c(OH-)�����ݵ���غ㣬��c(NH4��) ��c(Cl-)����˰�ˮ�ĵ���Ϊ������� c(NH4��) ��c(NH3��H2O)����C����D. 0.1 mol/LNaHA��Һ��pH=1��˵��HA����ȫ���룬����0.1 mol/L��pH=1��NaHA��Һ�м���NaOH��Һ��Ӧ�����ӷ���ʽΪ��H++ OH�� =H2O����D����ѡA��
��10-2mol/L��10-3mol/L��pH��3����B����C. 0.1 mol/L��ˮ��0.1 mol/L NH4Cl��Һ�������Ϻ�������ҺpH>7����c(H+)��c(OH-)�����ݵ���غ㣬��c(NH4��) ��c(Cl-)����˰�ˮ�ĵ���Ϊ������� c(NH4��) ��c(NH3��H2O)����C����D. 0.1 mol/LNaHA��Һ��pH=1��˵��HA����ȫ���룬����0.1 mol/L��pH=1��NaHA��Һ�м���NaOH��Һ��Ӧ�����ӷ���ʽΪ��H++ OH�� =H2O����D����ѡA��

 ��ҵ����ϵ�д�
��ҵ����ϵ�д� ͬ��ѧ��һ�ζ���ϵ�д�
ͬ��ѧ��һ�ζ���ϵ�д� �����ܾ�ϵ�д�
�����ܾ�ϵ�д� ���ƿ�����ϵ�д�
���ƿ�����ϵ�д�����Ŀ����������(���ӻ�����)������18�����ӣ�
���� | A�� | B2�� | C | D | E | F |
����ص� | �������� | �����˫�˷��� | ���ʣ�˫�˷��� | ��������˷��� | ������ĺ˷��� | |
��ش��������⣺
(1)A��Ԫ�ط�����____________��B2���Ľṹʾ��ͼ��________��
(2)C��D��ϣ�������Ӧ�Ļ�ѧ����ʽΪ_____________________________________��
(3)E�ĵ���ʽ��________��F��ˮ��Һ��������������F�Ļ�ѧʽ��________��