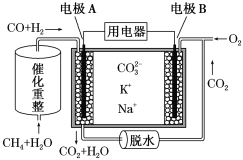
��Ŀ����
����Ŀ��ij�¶��£����ݻ�Ϊ1L���ܱ������г���1molCO2��3.25molH2������CO2(g)+3H2(g)![]() CH3OH(g)+H2O(g) ��H<0�����CH3OH�����ʵ�����ʱ��ı仯��ͼ��ʾ��(��֪�����¶��£��÷�Ӧ��ƽ�ⳣ��K=2.25)����˵����ȷ����
CH3OH(g)+H2O(g) ��H<0�����CH3OH�����ʵ�����ʱ��ı仯��ͼ��ʾ��(��֪�����¶��£��÷�Ӧ��ƽ�ⳣ��K=2.25)����˵����ȷ����
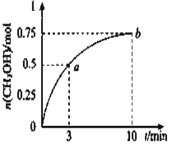
A.0-10min��v(H2)=0.075 mol/(L��min)
B.�� b ����Ӧ��״̬û�дﵽ��ѧƽ��״̬
C.CH3OH ���������ʣ��� a ���ڵ� b
D.������ƽ��״̬ʱ![]() ���ɱ��������������䣬�����¶�
���ɱ��������������䣬�����¶�
���𰸡�C
��������
A��0��10min��v(CH3OH)=  =0.075 molL-1min-1��v(H2)=3v(CH3OH)=3��0.075 molL-1min-1=0.225molL-1min-1����A����
=0.075 molL-1min-1��v(H2)=3v(CH3OH)=3��0.075 molL-1min-1=0.225molL-1min-1����A����
B��b��ʱ��n(CH3OH)=0.75mol��c(CH3OH)= ![]() =0.75mol/L����
=0.75mol/L���� b��Ũ����Qc=
b��Ũ����Qc=![]() =
=![]() =2.25����Qc=K=2.25��b��ﵽƽ��״̬����B����
=2.25����Qc=K=2.25��b��ﵽƽ��״̬����B����
C�����淴Ӧ��û�дﵽƽ��״̬ǰ�����ŷ�Ӧ�Ľ��У�����Ӧ������С������a��״����������ʴ���b�㣬��C��ȷ��
D���÷�Ӧ������Ӧ�Ƿ��ȷ�Ӧ�������¶�ƽ�������ƶ�����״�Ũ�ȼ�С��������̼Ũ����������![]() ��С����D����
������D����
��ΪC��

��ϰ��ϵ�д�
�����Ŀ