��Ŀ����
����Ŀ��̼���仯����㷺��������Ȼ���У��ش��������⣺
(1) ̼���γɻ�����ʱ��������Թ��ۼ�Ϊ����ԭ����___________________________��
(2) (CN)2�����У����ۼ���������________________��Cԭ�ӵ��ӻ����������____________
(3) CO�������Fe�γ�Fe(CO)5���û������۵�Ϊ253K���е�Ϊ376K�����������________���塣
(4) ��̼ͬ��Ļ�̬Geԭ�ӵĺ�������Ų�ʽΪ___________����________��δ�ɶԵ��ӡ�
(5) ʯī����������ӵ�صĸ������ϣ����ʱ����������Ӧ��Li1��xC6+xLi++xe��LiC6�������ǣ�Li+Ƕ��ʯī��A��B��䡣����ijʯīǶ�뻯����ÿ����Ԫ������Ӧһ��Li+��д�����Ļ�ѧʽ��_______��

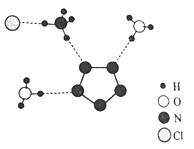
(6) ̼�ж���ͬ�������壬���н��ʯ�ľ��徧������ͼ��ʾ��
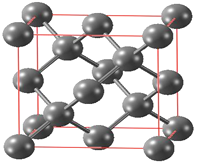
��֪���ʯ���������߳�a pm������㾧���ܶ�____________g/cm3(���ú�a��NA��ʽ�ӱ�ʾ)��
���𰸡� C��4���۵����Ұ뾶��С������ͨ���û�ʧ���Ӵﵽ�ȶ����ӽṹ �м��ͦҼ� sp ���Ӿ��� [Ar]3d104s24p2 2 LiC2 9.6��1031/(NA��a3)
�������� (1) ̼���γɻ�����ʱ��������Թ��ۼ�Ϊ����ԭ����C��4���۵����Ұ뾶��С������ͨ���û�ʧ���Ӵﵽ�ȶ����ӽṹ��
(2) (CN)2�����У����ۼ���������������������Cԭ�ӵ��ӻ����������sp��
(3) CO�������Fe�γ�Fe(CO)5���û������۵�Ϊ253K���е�Ϊ376K���������۵�ͷе�ϵͣ������ж���������ڷ��Ӿ�����
(4) ��̼ͬ��Ļ�̬Geԭ�ӵĺ�������Ų�ʽΪ[Ar]3d104s24p2����2��δ�ɶԵ��ӡ�
(5)ijʯīǶ�뻯����ÿ����Ԫ������Ӧһ��Li+������ÿ����Ԫ������6��Cԭ�ӣ���ÿ��Cԭ�Ӳ����γ�3����Ԫ��������ƽ��ÿ����Ԫ������2��Cԭ�ӣ����Ը�ʯīǶ�뻯����Ļ�ѧʽΪLiC2��
(6)�ɽ��ʯ�ľ����ṹʾ��ͼ��֪��ÿ����������8��Cԭ�ӣ���1mol��������8mol C��������Ϊ96g�������Ϊ![]() �����Ծ����ܶ�Ϊ
�����Ծ����ܶ�Ϊ 9.6��1031/(NA��a3) g/cm3��
9.6��1031/(NA��a3) g/cm3��

����Ŀ��úȼ���ŷŵ���������SO2��NO���γ����ꡢ��Ⱦ����������NaClO2��Һ��Ϊ���ռ���ͬʱ���������������������ش�����������
��1�ڹ��ݷ�Ӧ����ͨ�뺬�к���SO2��NO����������Ӧ�¶�Ϊ323 K��NaClO2��ҺŨ��Ϊ5��103mol��L1����Ӧһ��ʱ�����Һ������Ũ�ȵķ���������±���
���� | SO42 | SO32 | NO3 | NO2 | Cl |
c/��mol��L1�� | 8.35��104 | 6.87��106 | 1.5��104 | 1.2��105 | 3.4��103 |
��д��NaClO2��Һ������������Ҫ��Ӧ�����ӷ���ʽ__________________________������ѹǿ��NO��ת����______�����ߡ������䡱���͡�����
���������շ�Ӧ�Ľ��У����ռ���Һ��pH��______�����ߡ������䡱���͡�����
����ʵ������֪������Ӧ����______������Ӧ���ʣ�����ڡ���С�ڡ�����ԭ���dz���SO2��NO�������еij�ʼŨ�Ȳ�ͬ����������___________��
��2�ڲ�ͬ�¶��£�NaClO2��Һ���������ķ�Ӧ�У�SO2��NO��ƽ���ѹpx��ͼ��ʾ��
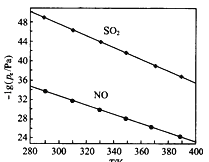
����ͼ������֪����Ӧ�¶����ߣ�����������Ӧ��ƽ�ⳣ����______________����������䡱��С������
�ڷ�ӦClO2+2SO32===2SO42+Cl��ƽ�ⳣ��K����ʽΪ___________��
��3���������NaClO��Ca(ClO)2���NaClO2��Ҳ�ܵõ��Ϻõ���������Ч����
�ٴӻ�ѧƽ��ԭ��������Ca(ClO)2���NaClO���е��е���_______��
����֪���з�Ӧ��
SO2(g)+2OH(aq)==SO32(aq)+H2O(l)��H1
ClO(aq)+SO32(aq)===SO42(aq)+Cl(aq)��H2
CaSO4(s)==Ca2+(aq)+SO42(aq)��H3
��ӦSO2(g)+Ca2+(aq)+ ClO(aq)+2OH(aq)===CaSO4(s)+H2O(l)+Cl(aq)�Ħ�H=______��