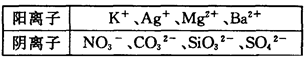��Ŀ����
ijУͬѧΪ̽��Br2��I2��Fe3����������ǿ��������������ʵ�顣
ʵ��٣�ȡ����KI��Һ���Թ��У��ȼ�����ˮ�����ټ���CCl4�����ã��۲쵽�²�Һ����Ϻ�ɫ��
ʵ��ڣ�ȡ����FeSO4��Һ���Թ��У��ȼ�����ˮ�����ټ����μ�����KSCN��Һ�����۲쵽��Һ�ʺ�ɫ��
��1��д�����ӷ���ʽ��
ʵ��٣�______________________________________________________��
ʵ��ڣ�______________________________________________________��
��2������������ʵ�飬�����ʵ������Կ��Եó�����ȷ������________��
| A��Br2>I2 | B��Fe3��>Br2 | C��Br2>Fe3�� | D��I��>Br�� |
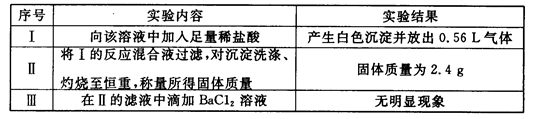
��FeCl3��Һ���ڵ�ˮ����KI��Һ����ϡH2SO4���ݵ�����Һ
��1����2I����Br2=I2��2Br������2Fe2����Br2=2Fe3����2Br������2��AC
��3��ȡ����FeCl3��Һ�ڽྻ�Թ��У����μ���KI��Һ�͵�����Һ��������Һ������֤��Fe3����������ǿ��I2��
����

�������ƣ�NaClO2��������ˮ��������ɰ�ǡ���֬��Ư����ɱ�����������ù������ⷨ�����������ƵĹ�������ͼ��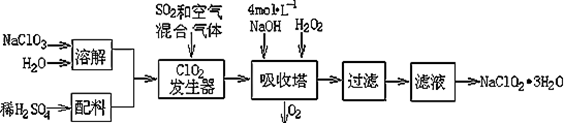
��֪����NaClO2���ܽ�����¶����߶������ʵ������¿ɽᾧ����NaClO2��3H2O ;
��Ksp(FeS)��6��3��10-18 �� Ksp(CuS)��6��3��10-36 ��Ksp(PbS)��2��4��10-28
��1���������ڷ�����Ӧ�����ӷ���ʽΪ ���ù��������е�NaClO3��ClO2��NaClO2����ǿ�����������Ƕ��ܺ�Ũ���ᷴӦ��ȡCl2�����ö������Ⱥ�Ũ������ȡCl2��������5 mol Cl2ʱ��ͨ����ԭ��Ӧ�Ƶ�����������Ϊ g��
��2������Һ�еõ�NaClO2��3H2O������������������ ����д��ţ���
a����b���գ�c���ˣ�d��ȴ�ᾧ��e����
��3��ӡȾ��ҵ�����������ƣ�NaClO2��Ư��֯�Ư��֯��ʱ���������õ���HClO2��
�±��� 25��ʱHClO2�����ֳ�������ĵ���ƽ�ⳣ����
| ���� | HClO2 | HF | HCN | H2S |
| Ka/mol?L-1 | 1��10-2 | 6��3��10-4 | 4��9��10-10 | K1��9��1��10?8 K2��1��1��10?12 |
�ٳ����£����ʵ���Ũ����ȵ�NaClO2��NaF��NaCN��Na2S������Һ��pH�ɴ�С��˳��Ϊ ���û�ѧʽ��ʾ���������ȣ����ʵ���Ũ����ͬ��NaF��NaCN����Һ�������������������Ĵ�С��ϵΪ�� ���ǰ�ߴ���ȡ����ߴ���
��Na2S�dz��õij�������ij��ҵ��ˮ�к��е�Ũ�ȵ�Cu2+��Fe2+��Pb2+���ӣ��μ�Na2S��Һ�����������ij����� �������һ�����ӳ�����ȫʱ��������Ũ��Ϊ10-5mol��L-1����ʱ��ϵ�е�S2-��Ũ��Ϊ ��
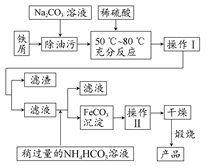

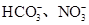 ����֪����Һ����Al2O3��Ӧ����
����֪����Һ����Al2O3��Ӧ����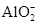 ���ɣ���ԭ��Һ��һ������_______�����ܺ��д�����_______��
���ɣ���ԭ��Һ��һ������_______�����ܺ��д�����_______�� Fe(SCN)2+ K1="200" ��Fe(SCN)2++SCN-
Fe(SCN)2+ K1="200" ��Fe(SCN)2++SCN-