ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩΚψΈ¬œ¬Θ§ΫΪa mol N2”κb mol H2ΒΡΜλΚœΤχΧεΆ®»κ“ΜΗωΙΧΕ®»ίΜΐΈΣ2LΒΡΟή±’»ίΤς÷–Θ§ΖΔ…ζ»γœ¬Ζ¥”ΠΘΚN2+ 3H2 ![]() 2NH3ΓΘ
2NH3ΓΘ
Θ®1Θ©»τΖ¥”ΠΫχ––5min ±Θ§≤βΒΟn(N2) = 1.8molΘ§n(NH3) = 0.4molΓΘ
ΦΤΥψΘΚΔΌaΒΡ÷ΒΘΜ ΔΎ”ΟH2≈®Ε»ΒΡ±δΜ·±μ ΨΒΡΖ¥”ΠΥΌ¬ ΓΘ
Θ®2Θ©Ζ¥”Π¥οΤΫΚβ ±Θ§ΜλΚœΤχΧεΒΡΉήΈο÷ ΒΡΝΩΈΣ5.0molΘ§Τδ÷–NH3ΒΡΚ§ΝΩΘ®ΧεΜΐΖ÷ ΐΘ©ΈΣ40%ΓΘ
ΦΤΥψΘΚ…œ ωΈ¬Ε»œ¬ΗΟΖ¥”ΠΒΡΜ·―ßΤΫΚβ≥Θ ΐΓΘ
ΓΨ¥πΑΗΓΩΘ®1Θ©ΔΌ2 ΔΎ0.06 mol/(LΓΛmin) Θ®2Θ©2
ΓΨΫβΈωΓΩ
‘ΧβΘ®1Θ© N2 + 3H2 ![]() 2NH3
2NH3
Τπ ΦΈο÷ ΒΡΝΩ a b 0
±δΜ·Έο÷ ΒΡΝΩ 0.2 0.6 0.4
5min ±Έο÷ ΒΡΝΩ 1.8 b-0.6 0.4
a=0.2+1.8=2 ”Ο«βΤχ≈®Ε»ΒΡ±δΜ·±μ ΨΒΡΖ¥”ΠΥΌ¬ ΈΣv (H2)=0.6molΓ¬(2LΓΝ5min)=0.06 mol/(LΓΛmin)
Θ®2Θ© N2 + 3H2 ![]() 2NH3
2NH3
Τπ ΦΈο÷ ΒΡΝΩ 2 b 0
±δΜ·Έο÷ ΒΡΝΩ 1 3 2
ΤΫΚβΈο÷ ΒΡΝΩ 1 b-3 2
1+2+b-3=5 ΫβΒΟb=5 K=Θ®1ΓΝ1Θ©Γ¬Θ®0.5ΓΝ13Θ©=2

ΓΨΧβΡΩΓΩΒΣΦΑΤδΜ·ΚœΈο»γNH3ΦΑοß―ΈΓΔN2H4ΓΔN2O4Β»‘Ύ÷–―ßΜ·―ßΓΔΜ·ΙΛΙΛ“ΒΓΔΙζΖάΒ»Νλ”ρ’Φ”–÷Ί“ΣΒΊΈΜΓΘ
(1)ΖΔ…δΚΫΧλΜπΦΐ≥Θ”Οκ¬(N2H4)”κN2O4Ής»ΦΝœ”κ÷ζ»ΦΦΝΓΘκ¬(N2H4)”κN2O4ΒΡΖ¥”ΠΈΣ
2N2H4 (g)+ N2O4(g)==3N2(g)+4H2O(g) ΓςH=-1077 kJΓΛmol-1ΓΘ
“―÷ΣœύΙΊΖ¥”ΠΒΡΜ·―ßΦϋΦϋΡή ΐΨί»γœ¬±μΥυ ΨΘΚ
Μ·―ßΦϋ | NΘ≠H | NΘ≠N |
| OΘ≠H |
E/(kJΓΛmolΘ≠1) | 390 | 190 | 946 | 460 |
ΔΌ Ι1 mol N2O4(g)Ζ÷Ή”÷–Μ·―ßΦϋΆξ»ΪΕœΝ― ±–η“ΣΈϋ ’ΒΡΡήΝΩ «________________ΓΘ
ΔΎœ¬Ν–ΡήΥΒΟς2N2H4 (g)+ N2O4(g)==3N2(g)+4H2O(g) ΓςH ¥οΤΫΚβΉ¥Χ§ΒΡ «________
a.ΜλΚœΤχΧεΒΡΤΫΨυœύΕ‘Ζ÷Ή”÷ ΝΩ≤Μ±δ b.VΘ®N2Θ©=3VΘ® N2O4Θ©
c.N2H4ΒΡ÷ ΝΩ±Θ≥÷≤Μ±δ d. ΓςH≤Μ‘Ό±δΜ·
(2)N2O4”κNO2÷°Φδ¥φ‘ΎΖ¥”ΠN2O4(g) ![]() 2NO2(g)ΓΘΫΪ“ΜΕ®ΝΩΒΡN2O4Ζ≈»ΥΚψ»ίΟή±’»ίΤς÷–Θ§≤βΒΟΤδΤΫΚβΉΣΜ·¬ [ΠΝ(N2O4)]ΥφΈ¬Ε»ΒΡ±δΜ·»γœ¬ΆΦΥυ ΨΓΘ
2NO2(g)ΓΘΫΪ“ΜΕ®ΝΩΒΡN2O4Ζ≈»ΥΚψ»ίΟή±’»ίΤς÷–Θ§≤βΒΟΤδΤΫΚβΉΣΜ·¬ [ΠΝ(N2O4)]ΥφΈ¬Ε»ΒΡ±δΜ·»γœ¬ΆΦΥυ ΨΓΘ

ΔΌ”…ΆΦΆΤ≤βΗΟΖ¥”ΠΒΡΓςH_______0(Χν>Γ±ΜρΓΑ<Γ±)Θ§άμ”…ΈΣ____________________________ΓΘ
ΔΎΆΦ÷–aΒψΕ‘”ΠΈ¬Ε»œ¬Θ§“―÷ΣN2O4ΒΡΤπ Φ―Ι«Ωp0ΈΣ108 kPaΘ§‘ρΗΟΈ¬Ε»œ¬Ζ¥”ΠΒΡΤΫΚβ≥Θ ΐKpΘΫ________________ (”ΟΤΫΚβΖ÷―Ι¥ζΧφΤΫΚβ≈®Ε»ΦΤΥψΘ§Ζ÷―Ι=Ήή―ΙΓΝΈο÷ ΒΡΝΩΖ÷ ΐ)ΓΘ
(3)ΒγΫβNO2÷Τ±ΗNH4NO3Θ§ΤδΙΛΉς‘≠άμ»γœ¬ΆΦΥυ ΨΓΘ

ΔΌ“θΦΪΒΡΒγΦΪΖ¥”Π ΫΈΣ____________________________________________________ΓΘ
ΔΎΈΣ ΙΒγΫβ≤ζΈο»Ϊ≤ΩΉΣΜ·ΈΣNH4NO3Θ§–η≤Ι≥δΡ≥÷÷Μ·ΚœΈοΓΣΓΣΈο÷ AΘ§‘ρAΒΡΜ·―ß ΫΈΣ________________ΓΘ
ΓΨΧβΡΩΓΩΡ≥ΖΦœψΧΰAΩ…“‘¥”ΟΚΗ…ΝσΒΟΒΫΒΡΟΚΫΙ”Ά÷–Ζ÷άκ≥ωά¥Θ§“‘AΈΣ‘≠ΝœΩ…“‘Κœ≥…ΨέΝΎΑ±Μυ±ΫΦΉΥαΓΔ±βΧ“ΥαΒ»Έο÷ Θ§ΤδΚœ≥…Νς≥Χ»γœ¬(≤ΩΖ÷≤ζΈοΓΔΚœ≥…¬ΖœΏΓΔΖ¥”ΠΧθΦΰ“―¬‘»Ξ)ΘΚ
“―÷ΣΘΚ
ΔώΘ°RΓΣCHO+HCN![]()
![]()
ΔρΘ°RΓΣCN![]() RΓΣCOOH
RΓΣCOOH
ΔσΘ°![]()
![]()
![]() (±ΫΑΖ“Ή±Μ―θΜ·)
(±ΫΑΖ“Ή±Μ―θΜ·)
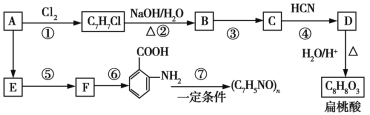
«κΜΊ¥πœ¬Ν–Έ ΧβΘΚ
(1)CΒΡΖ÷Ή” ΫΈΣ__________ΓΘ
(2)œ¬Ν–Ε‘œύΙΊΖ¥”Πάύ–ΆΒΡ≈–ΕœΚœάμΒΡ «__________ (Χν–ρΚ≈)ΓΘ
ΔΌ | ΔΎ | Δέ | Δή | Δί | Δό | ΔΏ | |
Δώ | Φ”≥… | Υ°Ϋβ | ΜΙ‘≠ | »Γ¥ζ | ΜΙ‘≠ | ―θΜ· | Φ”Ψέ |
Δρ | Φ”≥… | œϊ»Ξ | ΜΙ‘≠ | Φ”≥… | ―θΜ· | ΜΙ‘≠ | ΥθΨέ |
Δσ | »Γ¥ζ | Υ°Ϋβ | Φ”≥… | ―θΜ· | ΜΙ‘≠ | ΥθΨέ | |
Δτ | »Γ¥ζ | œϊ»Ξ | ―θΜ· | »Γ¥ζ | ΜΙ‘≠ | ―θΜ· | Φ”Ψέ |
(3)–¥≥ωΖ¥”ΠΔέΒΡΜ·―ßΖΫ≥Χ ΫΘΚ______________________________ΓΘ
(4)±βΧ“Υα”–Εύ÷÷Ά§Ζ÷“λΙΙΧεΘ§Τδ÷–Φ»Ρޔꬻ̷Χζ»ή“ΚΖΔ…ζœ‘…ΪΖ¥”ΠΘ§”÷Ρή”κΧΦΥα«βΡΤ»ή“ΚΖ¥”Π≤ζ…ζΤχ≈ίΒΡΆ§Ζ÷“λΙΙΧε”–__________÷÷Θ§–¥≥ωΤδ÷–“Μ÷÷ΒΡΫαΙΙΦρ ΫΘΚ__________________ΓΘ
(5)“‘ΖΦœψΧΰAΈΣ÷ς“Σ‘≠ΝœΘ§ΜΙΩ…“‘Ά®Ιΐœ¬Ν–Κœ≥…¬ΖœΏΚœ≥…ΑΔΥΨΤΞΝ÷ΚΆΕ§«ύ”ΆΘΚ

ΔΌΕ§«ύ”ΆΒΡΫαΙΙΦρ ΫΈΣ____________________ΓΘ
ΔΎ–¥≥ωΖ¥”ΠΔθΒΡΜ·―ßΖΫ≥Χ ΫΘΚ______________________________ΓΘ
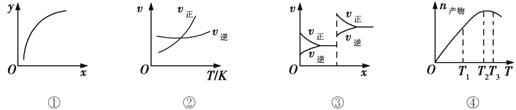
ΓΨΧβΡΩΓΩœ¬Ν–ΉΑ÷ΟΡή¥οΒΫ Β―ιΡΩΒΡΒΡ «
―Γœν | A | B | C | D |
Β―ιΉΑ÷Ο |
|
|
|
|
Β―ιΡΩΒΡ | ΧΫΨΩNa2CO3ΚΆNaHCO3»ήΫβΕ»ΒΡœύΕ‘¥σ–Γ | ”Ο“―÷Σ≈®Ε»ΒΡNaOH»ή“ΚΒΈΕ®Έ¥÷Σ≈®Ε»ΒΡœΓΝρΥα | Φλ―ι’αΧ«”κ≈®ΝρΥαΖ¥”Π≤ζ…ζΒΡCO2 | ―ι÷ΛΟΨΚΆœΓ―ΈΥαΒΡΖ¥”ΠΒΡ»»–ß”Π |
A. A B. B C. C D. D