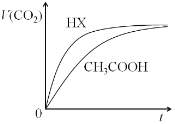
��Ŀ����
����Ŀ�����淴Ӧ3A(g)![]() 3B(?)+C(?) ��H>0�ﵽ��ѧƽ���
3B(?)+C(?) ��H>0�ﵽ��ѧƽ���
(1)�����¶ȣ����������������С������������������ȷ������ա�
a.��B��C�������壬�����ƽ����Է���������___��
b.��B��C���������壬�����ƽ����Է���������____��
c.��B�����壬C�������壬�����ƽ����Է���������___��
(2)���ƽ����¶Ȳ��䣬�������������һ������ƽ��ʱA��Ũ����ԭ����60%����B����___̬��C����___̬��
���𰸡���С ���� ��С ��̬��Һ̬ ��̬
��������
��1������Ӧ�����ȷ�Ӧ�������¶�ƽ��������Ӧ�����ƶ���
a.��B��C�������壬��Ӧ���������ģ����������ƽ����Է���������С��
b.��B��C���������壬�������ƽ����Է����������䣻
c.��B�����壬C�������壬����������ʵ������䣬��������С�����������ƽ����Է���������С��
��2���������������һ����˲�䣬A��Ũ����ԭ����50%��������ƽ��ʱA��Ũ����ԭ����60%��˵������ѹǿƽ�����淴Ӧ�����ƶ���������Ӧ�������С�ģ�����B��Һ̬���̬��C����̬��

 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�����Ŀ��ijͬѧ�о�FeSO4��Һ��AgNO3��Һ�ķ�Ӧ��������¶Ա�ʵ�顣
ʵ�� |
�� |
�� |
���� | ��ͨ��·������ָ������ƫת���ֱ�ȡ��Ӧǰ�ͷ�Ӧһ��ʱ�����ձ��е���Һ���μ�KSCN��Һ��ǰ������ɫ,�����Ժ�ɫ | ��ͨ��·������ָ��������С��ƫת���������ձ��о����������� |
����˵����ȷ����
A.���ɢ��е��������֪Ag+��������ǿ��Fe3+
B.���е�����ָ������ƫת��ԭ����Fe2+���������缫
C.�����������缫����ʯī�缫��������ָ����ܲ�������ƫת
D.�ԱȢ��֪������NO3��������Fe2+