��Ŀ����
����Ŀ�������£���0.1000mol��L��1NaOH��Һ�ζ�20.00mL0.1000mol��L��1CH3COOH��Һ���õζ�������ͼ������˵����ȷ����
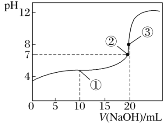
A.�����ʾ��Һ�У�c(CH3COOH)��c(Na��)��c(CH3COO��)
B.�����ʾ��Һ�У�c(Na��)��c(CH3COOH)��c(CH3COO��)
C.�����ʾ��Һ�У�c(OH��)��c(CH3COOH)��c(H��)
D.�ζ��п��ܳ��֣�c(CH3COO��)��c(H��)��c(OH��)��c(Na��)
���𰸡�C
��������
NaOH��Һ��Ũ���������ͬ���Ҵ�������Ϊ20mL�����Ԣٵ㣬��Һ��������Ũ�ȵĴ���ʹ����ƵĻ����Һ������ҺΪ���ԣ��۵�Ϊ�к͵ζ����յ㣬��Һ�����������Ƶ���Һ���ڵ����ҺpH=7�������ԣ�������Һ��![]() ��
��
A���ٵ����Һ�ǵ�Ũ�ȵĴ���ʹ����ƵĻ����Һ��������Һ�����ԣ�˵������ĵ���̶ȱȴ������ˮ��̶ȸ����ԣ�������Һ�У�![]() ��A�����
��A�����
B���ڵ����Һ![]() ��������Һ�ĵ���غ�ʽ��
��������Һ�ĵ���غ�ʽ��![]() ���ɵ���Һ�У�
���ɵ���Һ�У�![]() ��B�����
��B�����
C���۵����Һ�������Ƶ���Һ����������Һ�������غ�ʽΪ��![]() ��C����ȷ��
��C����ȷ��
D������Һ��![]() ������Һ��ɲ��غ㣬D�����
������Һ��ɲ��غ㣬D�����
��ѡC��

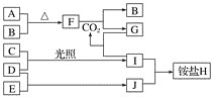
����Ŀ���������о������ʣ���H2 ���� ��Na2O2 ��CO2 ��H2SO4 ��Ba(OH)2���� �߰�ˮ ��ϡ���� ������Al2(SO4)3
��1�������ʵķ������д����Ŀհ״���
����� | �ܵ��� | �ǵ���� | ����� |
���ڸ�������� | ___ | ___ | ___ |
��2������ʮ������������������֮��ɷ������ӷ�Ӧ��H++OH-=H2O�������ӷ�Ӧ��Ӧ�Ļ�ѧ����ʽΪ___��
��3�������Ģߵμӵ������Һ�е����ӷ���ʽΪ___��34.2g������ˮ���250mL��Һ��SO42-�����ʵ���Ũ��Ϊ___��
��4������۳�ַ�Ӧ�Ļ�ѧ����ʽΪ��___����ת��NA���ӣ����ɱ������������Ϊ___��
��5����������Ӧ�Ļ�ѧ����ʽΪ��Al+4HNO3=Al(NO3)3+NO��+2H2O���÷�Ӧ����������___���ѧʽ������ԭ���������������ʵ���֮����___������5.4gAl������Ӧʱ��ת�Ƶ��ӵ����ʵ���Ϊ___��