��Ŀ����
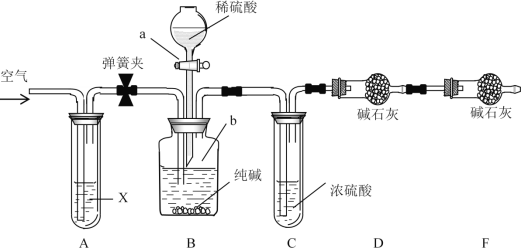
����Ŀ��(1)����A��B��C��D����ѧ�����Ļ���������ᴿ�Ļ���װ�á�
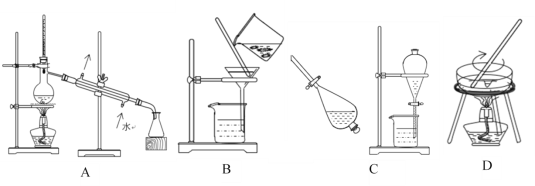
����ݻ���������ᴿ��ԭ�����ش��������⣺
���������ֻ�ѧʵ����������������ǣ�______��______��______��_______��
�ڷ���CCl4(�е㣺76.75��)�ͱ�(�е㣺110.6��)�Ļ����(����)Ӧѡ��________��(��װ����ĸ)
����ʹ��C���л�ѧʵ�����ǰ��Ӧ��____________��
(2)ʵ��������Ҫ480ml 0.10mol/L��NaOH��Һ�������ø���Һ�ش��������⣺
����������ƽ����NaOH����___________g��
�ڳ�������Ѹ�٣�ԭ����__________________________________________��
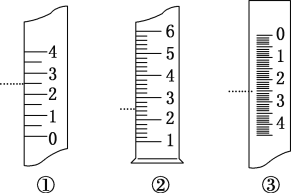
�۽����úõ���Һȷȡ��480ml��ʣ����Һȡ����ˮϡ����100ml�������Լ�ƿ�У���������ϱ�ǩ����ǩ�ϵ�������__________________��
���𰸡����� ���� ��ȡ����Һ ���� A ����Һ©���Ƿ�©Һ 2.0 ��ֹNaOH�ܳ������ҺŨ�Ȳ�ȷ 0.02mol/L NaOH��Һ
��������
(1)�ٸ���ͼʾ��֪��������װ��ͼ�ֱ��ʾ���������ˡ���ȡ�ͷ�Һ�������������Ȼ�̼�ͱ��ǻ��ܵ�Һ��������ڶ��߷е����ϴ����Կ���������ķ������룬��ȷѡ��ΪA��
�۷�Һ©��������ȡʱҪ�����װ�õ��ܱ���Ҫǿ��������ʹ�������л�ѧʵ�����ǰ��Ӧ�ȼ���Һ©���Ƿ�©Һ��
(2)��ʵ��������Ҫ480ml 0.10mol/L��NaOH��Һ������û��480mL��������ƿ������ѡ��������ԭ���ǡ����������Ҫѡ��500mL����ƿ������Һ��n(NaOH)=cV=0.10mol/L��0.5L=0.050mol��������������ƽ����NaOH��������m(NaOH)=0.050mol��40g/mol=2.0g��
��NaOH������������տ����е�ˮ���������⣬���Գ�������Ѹ�٣�
��������Һ��Ũ����ȡ������Һ�������С�أ�����Һ���о�һ�ԣ����Ա�ǩ��ע����Һ��Ũ�Ⱥ����ʳɷּ��ɣ����DZ�ǩ��������0.02mol/L NaOH��Һ��

����Ŀ��I.һ������CO(g)��H2O(g)�ֱ�ͨ���ݻ�Ϊ1L�ĺ����ܱ������У�������ӦCO(g)+H2O![]() CO2(g)+H2(g)�õ������������ݣ�
CO2(g)+H2(g)�õ������������ݣ�
ʵ���� | �¶ȡ� | ��ʼ��/mol | ƽ����/mol | �ﵽƽ�n����ʱ��/min | ||
CO | H2O | CO2 | ||||
1 | 500 | 8 | 4 | 3.2 | 4 | |
2 | 700 | 4 | 2 | 0.8 | 3 | |
3 | 700 | 4 | 2 | 0.8 | 1 | |
(1)�������������Ӧ�ﵽƽ��״̬����________(�����)
A. CO2��H2�����������
B.�¶Ȳ���ʱ��ѹǿ����
C.����CO2�����ʺ�����CO���������
(2)ʵ��2�У���Ӧ�ﵽƽ��״̬ʱ��CO��ת����Ϊ__________��
(3)ʵ��3��ʵ��2��ȣ��ı��������_____________��
II.(1)������Һ���н�ǿ���ԣ���ԭ����___________(�����ӷ���ʽ��ʾ)��
(2)��ʹ0.1mol��Na2S��Һ�У� 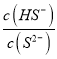 ��ֵ���ɲ�ȡ�Ĵ�ʩ��___________(�����)
��ֵ���ɲ�ȡ�Ĵ�ʩ��___________(�����)
A.��������Ũ�ȵ�NaOH��Һ
B.��������ˮ
C.ͨ��������H2S����