��Ŀ����
ͭ����Ҫ�Ľ������ϡ�
(1)��ҵ�Ͽ���Cu2S��O2��Ӧ��ȡ��ͭ���÷�Ӧ��������Ϊ _______ ������ͭ��ȡ��ͭ�����ʱ������������ _______ �����Һ�б��뺬�е��������� _______ ��
(2)��100 mL 18 mol/LŨ�����м��������ͭƬ������ʹ֮��ַ�Ӧ����Ӧ�б���ԭ��H2SO4< __ mol��
(3)���ӹ�ҵ����30����FeCl3��Һ��ʴ����ͭ���ľ�Ե����ӡˢ��·�壬Ϊ�˴�ʹ�ù��ķϸ�ʴҺ�л���ͭ������н�õ�FeCl3��Һ���������ʵ�����̡�
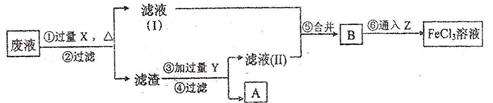
���������У������Լ��Ļ�ѧʽΪ��X _______ ��Y _______ Z _______ ���ڢ���Ӧ�����ӷ���ʽΪ _______ ��
(1)��ҵ�Ͽ���Cu2S��O2��Ӧ��ȡ��ͭ���÷�Ӧ��������Ϊ _______ ������ͭ��ȡ��ͭ�����ʱ������������ _______ �����Һ�б��뺬�е��������� _______ ��
(2)��100 mL 18 mol/LŨ�����м��������ͭƬ������ʹ֮��ַ�Ӧ����Ӧ�б���ԭ��H2SO4< __ mol��
(3)���ӹ�ҵ����30����FeCl3��Һ��ʴ����ͭ���ľ�Ե����ӡˢ��·�壬Ϊ�˴�ʹ�ù��ķϸ�ʴҺ�л���ͭ������н�õ�FeCl3��Һ���������ʵ�����̡�

���������У������Լ��Ļ�ѧʽΪ��X _______ ��Y _______ Z _______ ���ڢ���Ӧ�����ӷ���ʽΪ _______ ��
��1��Cu2S ��O2����ͭ �� Cu2+
��2��0.9
��3��X-Fe ��Y- HCl�� Z- Cl2�� 2Fe2+��Cl2��2Fe3+��2Cl��
��2��0.9
��3��X-Fe ��Y- HCl�� Z- Cl2�� 2Fe2+��Cl2��2Fe3+��2Cl��
��2����������1.8mol�����÷�Ӧ�����������ֹͣ��ʵ�ʲ��뷴Ӧ������С��1.8mol,������ԭ��������ֻ�Dzμӷ�Ӧ�����һ�룬�ʱ���ԭ������С��0.9mol��

��ϰ��ϵ�д�
 С��ſ�ʱ��ҵϵ�д�
С��ſ�ʱ��ҵϵ�д� һ������ϵ�д�
һ������ϵ�д� �Ƹ�С״Ԫ���ֳ������ϵ�д�
�Ƹ�С״Ԫ���ֳ������ϵ�д� �¸��̵�ѧϵ�д�
�¸��̵�ѧϵ�д� ����ͬѧһ����ʦȫ�źþ�ϵ�д�
����ͬѧһ����ʦȫ�źþ�ϵ�д�
�����Ŀ
 Cu2S+3SO2+2FeO(¯��)��
Cu2S+3SO2+2FeO(¯��)��
