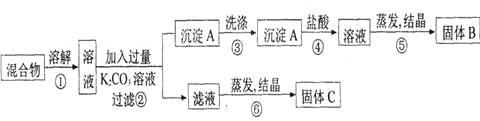
��Ŀ����
����Ŀ�� I.����������ԭ��ӦZn(s)��Cu2��(aq)===Zn2��(aq)��Cu(s)��Ƶ�ԭ�����ͼ��ʾ��
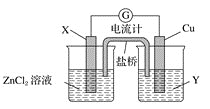
��ش��������⣺
(1)ͭ�缫Ϊ��ص�________�����������ҺY��________________��
(2)�����е�Cl����________��������ҡ����ƶ����罫���ų����������Ƶ�ָ�뽫��ƫת��
���ԭ���ڻ�ѧ��ҵ���й㷺Ӧ�á�
��ͼ��ʾһ�����أ�װ�е��Һa��X��Y������缫�壬ͨ��������ֱ����Դ������������������⣺
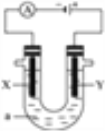
(1)��X��Y���Ƕ��Ե缫��a�DZ���NaCl��Һ��ʵ�鿪ʼʱ��ͬʱ�����߸����뼸�η�̪��Һ��
����X�������۲쵽��������_________________��
�ڵ��һ��ʱ��÷�Ӧ�����ӷ���ʽ_______________________________��
(2)���ø�װ�õ�⾫��ͭ�����Һaѡ��CuSO4��Һ����X�缫�IJ�����____________���һ��ʱ���CuSO4��ҺŨ��_________���������С�����䡱����
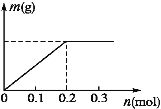
(3)��X��Y���Ƕ��Ե缫��a������ΪCu(NO3)2��X(NO3)3���Ҿ�Ϊ0.1 mol�Ļ����Һ��ͨ��һ��ʱ�������������������m(g)��ͨ�����ӵ����ʵ���n(mol)��ϵ��ͼ��ʾ����Cu2+��X3+��H+���������ɴ�С��˳����_____��
���𰸡��� CuSO4 �� �������������Һ��� 2Cl-+2H2O ![]() 2OH-+Cl2��+H2�� ��ͭ ��С Cu2+��H+ ��X3+
2OH-+Cl2��+H2�� ��ͭ ��С Cu2+��H+ ��X3+
��������
I.ԭ����нϻ��õĽ����Ǹ�����ʧȥ���ӣ�����������Ӧ�����Ӿ����ߴ��ݵ���������Һ�е��������������ƶ��������õ����ӣ�������ԭ��Ӧ���ݴ˷������
II����1����ⱥ��ʳ��ˮʱ����������������ʧ���ӣ��������������ӵõ��ӣ�����������Ũ�Ȼ��С��������ǿ���ݴ˻ش�
��2����⾫��ͭʱ���ص�����X�����Ǵ�ͭ�������Ǵ�ͭ�����бȽ���ͭ���õĽ������ȷŵ磻
��3�����ݵ���ͼ���֪��ͨ�����й������ɣ���ͨ������Ϊ0.2molʱ�������������������֤����ʱ�����Ĺ�����ͭ�������X3+������������Ӧ����0.3mol�������ӳ���0.2molʱ����������û�䣬˵�������������������������ˮ���ݴ˻ش�����������
I.��1�����ݷ���ʽZn(s)��Cu2��(aq)��Zn2��(aq)��Cu(s)��֪п�ǻ�ԭ������Һ�е�ͭ���ӵõ����ӣ���X��п�缫��Ϊ������ͭ�缫Ϊ��ص��������������ҺY��CuSO4��
��2�������е�Cl�������������ƶ���
��1���ٺ͵�Դ�ĸ��������ĵ缫X�����������õ缫�������ӷ����õ��ӵĻ�ԭ��Ӧ����2H++2e-��H2�������Ըõ缫����������Ũ����������ǿ�����뼸�η�̪��Һ���죬����X�������۲쵽���������������������Һ��죻
�ڵ�ⱥ��ʳ��ˮ�õ��IJ������������ơ���������������Ӧ�����ӷ���ʽΪ2Cl-+2H2O![]() 2OH-+Cl2��+H2����
2OH-+Cl2��+H2����
��2����⾫��ͭʱ���ص�����X�����Ǵ�ͭ���缫��ӦΪCu2++2e-��Cu�������Ǵ�ͭ���缫��ӦΪ��Cu-2e-��Cu2+�����бȽ���ͭ���õĽ������ȷŵ磬���Ե��һ��ʱ���CuSO4��ҺŨ�Ȼή�ͣ�
��3�����ݵ���ͼ���֪��ͨ�����й������ɣ���ͨ������Ϊ0.2molʱ�������������������֤����ʱ�����Ĺ�����ͭ�������X3+������������Ӧ����0.3mol������������ΪCu2+��X3+�������ӳ���0.2molʱ����������û�䣬˵�������������������������ˮ��˵����������H+��X3+������������ΪCu2+��H+��X3+��

 �Ͻ�ƽСѧ��������ϵ�д�
�Ͻ�ƽСѧ��������ϵ�д�����Ŀ�����и��������У���������ͼ��ʾת����ϵ���ǣ���Ӧ������ȥ����ͷ��ʾһ��ת������ ��
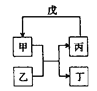
ѡ�� | �� | �� | �� | �� |
A | NH3 | Cl2 | N2 | H2 |
B | C | SiO2 | CO | CuO |
C | Al��OH��3 | NaOH | NaAlO2 | CO2 |
D | Br2 | FeI2 | FeBr3 | Cl2 |
A. AB. BC. CD. D
����Ŀ���ߴ���������Ϊ�ϳ���������Ԫ�������ϵ�ԭ�ϣ���ҵ�Ͽ�����Ȼ�������̷������̿���Fe��Al��Mg��Zn��Ni��Si��Ԫ�أ��Ʊ�����������ͼ��ʾ���ش��������⣺
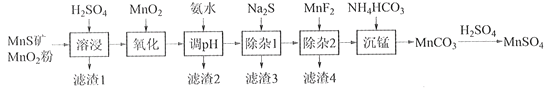
��ؽ�������[c0(Mn+)=0.1 mol��L1]�γ��������������pH��Χ���£�
�������� | Mn2+ | Fe2+ | Fe3+ | Al3+ | Mg2+ | Zn2+ | Ni2+ |
��ʼ������pH | 8.1 | 6.3 | 1.5 | 3.4 | 8.9 | 6.2 | 6.9 |
������ȫ��pH | 10.1 | 8.3 | 2.8 | 4.7 | 10.9 | 8.2 | 8.9 |
��1��������1������S��__________________________��д�����ܽ����ж������������̷�Ӧ�Ļ�ѧ����ʽ____________________________________________________��
��2����������������������MnO2�������ǽ�________________________��
��3������pH��������������Һ��pH��ΧӦ����Ϊ_______~6֮�䡣
��4��������1����Ŀ���dz�ȥZn2+��Ni2+��������3������Ҫ�ɷ���______________��
��5��������2����Ŀ��������MgF2������ȥMg2+������Һ��ȹ��ߣ�Mg2+��������ȫ��ԭ����_____________________________________________________________________��
��6��д���������������ӷ���ʽ___________________________________________________��
��7����״��������Ԫ���Ͽ���Ϊ����ӵ���������ϣ��仯ѧʽΪLiNixCoyMnz2������Ni��Co��Mn�Ļ��ϼ۷ֱ�Ϊ+2��+3��+4����x=y=![]() ʱ��z=___________��
ʱ��z=___________��