��Ŀ����
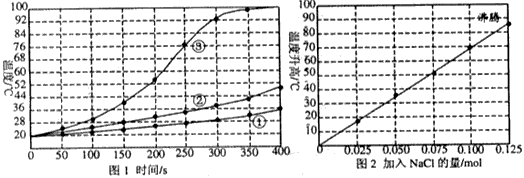
����ʳƷ����������ʿ������Ұ�����ʳ����ijɷ���þ�ۡ����ۡ��Ȼ��ƣ�ʹ��ʱ����ˮ����������þ��ˮ��Ӧ�����ģ�1.0gþ��ˮ��Ӧ�ų�����14.7kJ�������ǶԸò�Ʒ��ʵ���о���ʵ��1�������½�1.0molþ����0.10mol���ۺ�0.10mol���Ȼ��Ƽ������������ˮ�У��������裬ÿ50s��¼һ���¶���ͼ1�ϣ�����1�����ٰ�1.0molþ���ֳ�100�ݣ�����2����1.0molþ�ۣ�����3������þ���ظ�����ʵ�飮

ʵ��2����0.10molþ�ۺ�0.10mol�Ȼ��ƻ�ϣ������·������������100mLˮ�У����Ͻ��裬15minʱ��¼�¶ȣ�����������ͬʱ�¶ȱ仯�����ͼ2��ʾ

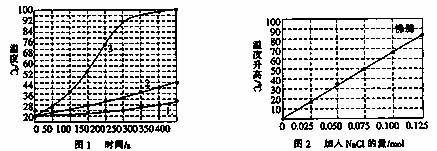
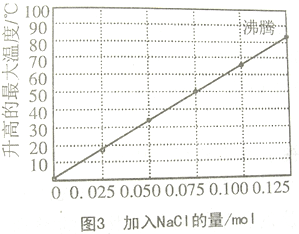
ʵ��3����0.10molþ�ۺ�0.50mol���ۻ�ϼ������������100mLˮ�У����Ͻ��裬15minʱ��¼�¶ȣ��Ȼ���������ͬʱ�¶ȱ仯�����ͼ3��ʾ��
��ش�
��1��ʵ��1���Է���Ӱ��þ��ˮ��Ӧ���ʵ�������
A����Ӧ�¶� B��NaCl���� C�������� D��þ�ķ�Ӧ�����
��2��ʵ��3�д���0.125molNaCl��������ʵ��Ͳ�������ԭ����
A���������NaCl�������ӷ�Ӧ���� B������NaCl�ή�ͷ�Ӧ����
C���Ѵﵽ�е㲻�������¶ȱ仯 D����Ҫ����������������¶�
��3�������ʵ��3�м�����0.060mol��NaCl���������¶���ӽ�
A��34��B��42��C��50��D��62��
��4��һλ����ʦ���������ʳ�������ʱ��ϣ���þ������ٵ����ϲ��������ܶ����������������ʵ��Ӧѡ��������
A��0.50mol�����ۡ�0.125molNaCl��0.10mol��þ��
B��0.50mol�����ۡ�0.125molNaCl��0.10mol��þ��
C��0.70mol�����ۡ�0.125molNaCl��0.10mol��þ��
D��0.70mol�����ۡ�0.125molNaCl��0.10mol��þ��
��5��д���������������Ӧ���Ȼ�ѧ����ʽ
��6������Ϊ���ۡ�NaClΪʲô��ʹ��Ӧ��������
��������1��þ��ˮ��ӦΪ���ȷ�Ӧ����������1������2������3��ֻ��þ��״̬��ͬ�����жϣ�
��2������ͼ3���Ȼ��Ƶ����ʵ�������0.125molʱ��Һ�Ѿ����ڷ�����
��3������ʵ��3��ͼ���֪������0.060mol��NaCl����Ӧ�¶���40-50��֮�䣻
��4��þ�۱�þ�������������죬����ʵ��2��֪��0.10molþ�۴���ʱ������0.50mol�����Ѿ��ﵽ����¶ȣ�
��5������1.0gþ��ˮ��Ӧ�ų�����14.7kJд���������������Ӧ���Ȼ�ѧ����ʽ��
��6�������γ���ԭ��أ��ܹ��ӿ췴Ӧ���ʽ��з�����
��2������ͼ3���Ȼ��Ƶ����ʵ�������0.125molʱ��Һ�Ѿ����ڷ�����
��3������ʵ��3��ͼ���֪������0.060mol��NaCl����Ӧ�¶���40-50��֮�䣻
��4��þ�۱�þ�������������죬����ʵ��2��֪��0.10molþ�۴���ʱ������0.50mol�����Ѿ��ﵽ����¶ȣ�
��5������1.0gþ��ˮ��Ӧ�ų�����14.7kJд���������������Ӧ���Ȼ�ѧ����ʽ��
��6�������γ���ԭ��أ��ܹ��ӿ췴Ӧ���ʽ��з�����
����⣺��1��1.0gþ��ˮ��Ӧ�ų�����14.7kJ����Ӧ����Խ�죬�ų�������Խ����Һ���¶�Խ�ߣ���������1��2��3��ֻ��þ�Ĺ��ͬ������Ӱ��þ��ˮ��Ӧ���ʵ������ǣ�þ�ķ�Ӧ�������
�ʴ�Ϊ��D��
��2������ͼ3��ͼ���֪������0.125mol�Ȼ��ƺ���Һ�Ѵﵽ�е㲻�������¶ȱ仯�����Դ���0.125molNaCl��������ʵ��Ͳ������ˣ�
�ʴ�Ϊ��C��
��3����ͼ3��֪��������0.060mol��NaCl����Ӧ�¶ȴ���40�棬С��50�棬�������¶���ӽ�42�棬
�ʴ�Ϊ��B��
��4��þ�۱�þ���ܸ��������������þ���Ȼ��ƹ̶�������£�0.1molþ�м���0.50mol����ʱ��Ӧ�¶ȴﵽ����ټ�����������ۻ�����˷ѣ�����A��ȷ��
�ʴ�Ϊ��A��
��5������1.0gþ��ˮ��Ӧ�ų�����14.7kJ��24gþ�ų�������Ϊ352.7kJ���������������Ӧ���Ȼ�ѧ����ʽΪ��Mg��s��+2H2O��l��=Mg��OH��2��s��+H2��g����H=-352.8kJ/mol��
�ʴ�Ϊ��Mg��s��+2H2O��l��=Mg��OH��2��s��+H2��g����H=-352.8kJ/mol��
��6��þ�����ۺ�NaCl��Һ������ԭ��أ��ܹ�ʹ��Ӧ���ʴ��ӿ죬�ʴ�Ϊ��������þ�����ۺ�NaCl������ԭ��أ�
�ʴ�Ϊ��D��
��2������ͼ3��ͼ���֪������0.125mol�Ȼ��ƺ���Һ�Ѵﵽ�е㲻�������¶ȱ仯�����Դ���0.125molNaCl��������ʵ��Ͳ������ˣ�
�ʴ�Ϊ��C��
��3����ͼ3��֪��������0.060mol��NaCl����Ӧ�¶ȴ���40�棬С��50�棬�������¶���ӽ�42�棬
�ʴ�Ϊ��B��
��4��þ�۱�þ���ܸ��������������þ���Ȼ��ƹ̶�������£�0.1molþ�м���0.50mol����ʱ��Ӧ�¶ȴﵽ����ټ�����������ۻ�����˷ѣ�����A��ȷ��
�ʴ�Ϊ��A��
��5������1.0gþ��ˮ��Ӧ�ų�����14.7kJ��24gþ�ų�������Ϊ352.7kJ���������������Ӧ���Ȼ�ѧ����ʽΪ��Mg��s��+2H2O��l��=Mg��OH��2��s��+H2��g����H=-352.8kJ/mol��
�ʴ�Ϊ��Mg��s��+2H2O��l��=Mg��OH��2��s��+H2��g����H=-352.8kJ/mol��
��6��þ�����ۺ�NaCl��Һ������ԭ��أ��ܹ�ʹ��Ӧ���ʴ��ӿ죬�ʴ�Ϊ��������þ�����ۺ�NaCl������ԭ��أ�
���������⿼����̽�����ȷ�Ӧ�ͷ��ȷ�Ӧ���Ȼ�ѧ����ʽ����д����Ŀ�Ѷ��еȣ������Ǹ߿��еij������ͣ������ۺ���ǿ������������ѧ������˼ά�����ͷ�ɢ˼ά���������ѧ����Ӧ��������ѧϰЧ�ʣ�

��ϰ��ϵ�д�
�����Ŀ