��Ŀ����
9����1�������£�0.1mol•L-1CuSO4��Һ�и�����Ũ���ɴ�С��˳����c��SO42-����c��Cu2+����c��H+����c��OH-������2��Na2CO3��Һ�ɴ���ϴ�Ӽ�ϴ�Ӳ;ߣ�ԭ���ǣ������ӷ���ʽ��ʾ��CO32-+H2O?HCO3-+OH- HCO3-+H2O?H2CO3+OH-����ͬ���ʵ���Ũ�ȵ�Na2CO3��Һ��NaHCO3��Һ��pH��С��ǰ�ߣ� ���ߣ����=����
��3����ͨ��ĭ�����������NaHCO3��Һ��Al2��SO4��3��Һ��ϣ���������������ͳ��������彫�����ѹ�����������ط�Ӧ�����ӷ���ʽ��Al3++3HCO3-=Al��OH��3��+3CO2����
��4�������£�Cr��OH��3���ܶȻ�Ksp=c��Cr3+��•c3��OH-��=10-32��Ҫʹc��Cr3+������10-5mol/L����Һ��pHӦ����5��
���� ��1������ͭ��ͭ���ӿ��Է���ˮ�ⷴӦ����Һ��ʾ���ԣ�
��2������ɴ���ϴ�Ӽ�ϴ�Ӳ;�����Ϊ̼����ˮ��Һ��ˮ���Լ��ԣ���֬�ڼ���Һ��ˮ����������ˮ������ϴȥ��̼������ӵ�ˮ��̶ȴ���̼��������ӵ�ˮ��̶ȣ�
��3����������̼������ˮ��Һ�з���˫ˮ�������������������Ͷ�����̼��
��4�������ܶȻ������Լ�ˮ�����ӻ����������м��㣮
��� �⣺��1������ͭ��ͭ���ӿ��Է���ˮ�ⷴӦ������c��SO42-����c��Cu2+������Һ��ʾ���ԣ�����c��H+����c��OH-��������Һ�и�����Ũ���ɴ�С��˳����c��SO42-����c��Cu2+����c��H+����c��OH-�����ʴ�Ϊ��c��SO42-����c��Cu2+����c��H+����c��OH-����
��2������ɴ���ϴ�Ӽ�ϴ�Ӳ;�����Ϊ̼����ˮ��Һ��ˮ���Լ��ԣ���֬�ڼ���Һ��ˮ����������ˮ������ϴȥ����Ӧ�����ӷ���ʽΪ��CO32-+H2O?HCO3-+OH- HCO3-+H2O?H2CO3+OH-��̼������ӵ�ˮ��̶ȴ���̼��������ӵ�ˮ��̶ȣ�����̼���Ƶļ���ǿ��̼�����ƣ�����ǿ��pH��
�ʴ�Ϊ��CO32-+H2O?HCO3-+OH- HCO3-+H2O?H2CO3+OH-������
��3����������̼������ˮ��Һ�з���˫ˮ�������������������Ͷ�����̼����Ӧ�����ӷ���ʽΪ��Al3++3HCO3-=Al��OH��3��+3CO2����
�ʴ�Ϊ��Al3++3HCO3-=Al��OH��3��+3CO2����
��4����c��Cr3+��=10-5mol/Lʱ����Һ��c��OH-��=$\root{3}{\frac{1{0}^{-32}}{1{0}^{-5}}}$=10-9 mol/L��c��H+���T$\frac{1{0}^{-14}}{1{0}^{-9}}$=10-5mol/L��pH=5����Ҫʹc��Cr3+������10-5mol/L����Һ��pHӦ����5���ʴ�Ϊ��5��
���� ���⿼��������Һ������Ũ�ȵĹ�ϵ�������ܽ�ƽ�⡢˫ˮ�ⷴӦ��֪ʶ�㣬�ѶȽϴ�ע��֪ʶ�Ĺ��ɺ������ǹؼ���

 �����ܿ����ϵ�д�
�����ܿ����ϵ�д�
��ѡ�õ��Լ����£�
A��ʳ�ξ��塡 Bˮ�� C������ʳ��ˮ�� D���ռ���Һ�� E������
F���������̡� G��Ũ���ᡡ H����ˮCaCl2�� I����ʯ��
��1��������������ѡȡ��������һ���Ʊ���������ˮFeCl3��װ�ã���ͼ�и��ܿڱ�Ű��Ⱥ������Ϊd��e��f��g��h��a��b��c��
��2����д����װ���и�ѡ��������Ӧʢ�ŵ����ʣ�
| ������� | |||||
| �Լ���� |
| A�� | ���ܽ⣬��ͭ��������������� | B�� | ֻ��������� | ||
| C�� | ֻ�г������� | D�� | �������������������ɫ�������� |
| A�� | HCO3- | B�� | SO32- | C�� | CO32- | D�� | CH3COO- |
| A�� | ʯīת��Ϊ���ʯ�����ڻ�ѧ�仯 | B�� | ʯī�Ƚ��ʯ���ȶ� | ||
| C�� | 1molʯī��1mol���ʯ���������� | D�� | ʯī�ͽ��ʯ��̼Ԫ�ص�ͬλ�� |
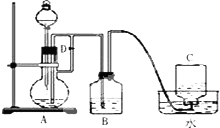 ����Fe��OH��2�ڿ������ױ�������Ϊ�˹۲쵽��ɫ��Fe ��OH��2���˺ܶ����
����Fe��OH��2�ڿ������ױ�������Ϊ�˹۲쵽��ɫ��Fe ��OH��2���˺ܶ����
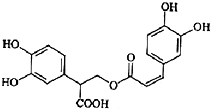 �Ե������Ǵӷ仨��ֲ������ȡ�õ����������ʣ���ṹ��ͼ��
�Ե������Ǵӷ仨��ֲ������ȡ�õ����������ʣ���ṹ��ͼ��