��Ŀ����
����Ŀ�������[��NH4)2C2O4] Ϊ��ɫ��״���壬���ȶ� �������ֽ⣬�����ڲⶨ Ca2+��Mg2+�ĺ�����
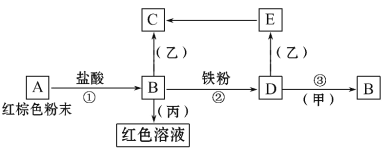
I.ijͬѧ������ͼ��ʾʵ��װ�ü������淋ķֽ���

��1��ʵ������У��۲쵽���з�̪��Һ����ֽ��죬װ�� B �г���ʯ��ˮ����ǣ�˵���ֽ�����к���__________________���ѧʽ�������۲쵽__________________��˵���ֽ�����к��� CO������立ֽ�Ļ�ѧ����ʽΪ______________________��
��2����Ӧ��ʼǰ ��ͨ�뵪����Ŀ����________________________��
��3��װ�� C ��������_______________________��
��4������һ�ַֽ������һ��������Ҳ�ܻ�ԭCuO , �÷�Ӧ�Ļ�ѧ����ʽΪ__________��
II.��ͬѧ���ò���鱗ⶨѪҺ�и�Ԫ�صĺ��� ��
��5��ȡ 20.00 mL ѪҺ��Ʒ �������� l00m L, �ֱ�ȡ���������Ϊ25.00 mL ϡ�ͺ��ѪҺ��Ʒ���������泥����ɲ���Ƴ��������ˣ����ó������ڹ���ϡ�����У�Ȼ���� 0.0l00mol/L KMnO4 ��Һ���еζ����ζ����յ�ʱ��ʵ������Ϊ___________�����εζ�ʵ������ KMnO4 ��Һ������ֱ�Ϊ0.43mL , 0.41 m L , 0.52mL, ���ѪҺ��Ʒ�и�Ԫ�صĺ���Ϊ________m mol/L��
���𰸡�![]() ��
��![]() װ��E������ͭ�ɺ�ɫ��Ϊ��ɫ��װ��F�г���ʯ��ˮ�����
װ��E������ͭ�ɺ�ɫ��Ϊ��ɫ��װ��F�г���ʯ��ˮ����� 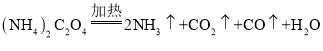 �ž�װ���ڵĿ���������CO�������ϼ��ȷ�����ը������ֹ�����е�
�ž�װ���ڵĿ���������CO�������ϼ��ȷ�����ը������ֹ�����е�![]() ����ʵ�� ����
����ʵ�� ����![]() �������CO�ļ����������
�������CO�ļ���������� 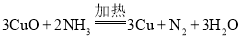 ���������һ��
���������һ��![]() ��Һʱ����Һ����ɫ��Ϊdz��ɫ���Ұ�����ڲ���ɫ 2.10
��Һʱ����Һ����ɫ��Ϊdz��ɫ���Ұ�����ڲ���ɫ 2.10
��������
I���������A�����ȷֽ⣬���������а�����������ˮ��Ӧ���ɰ�ˮ����ˮ�Լ��ԣ�����з�̪��Һ����ֽ��죬ͨ�����ʯ��ˮ��������ʯ��ˮ����ǣ�������ж�����̼����NaOH��Һ��ȥ������̼����Ũ�����ȥˮ���������������������ڱ�죬��F�г���ʯ��ˮ�������֤����������CO��
II�������ӺͲ�����������ɲ���Ƴ���������ƺ����ᷴӦ��������ƺͲ��ᣬ�ø�����صζ�����Ӷ���ӵζ������ӡ�
��.��1��ʵ������У��۲쵽���з�̪��Һ����ֽ��Ϊ��ɫ˵���ֽ�����к��а�����װ��B�г���ʯ��ˮ����ǣ�˵���ֽ�����к��ж�����̼���壬���۲쵽װ��E������ͭ�ɺ�ɫ��Ϊ��ɫ��װ��F�г���ʯ��ˮ����ǣ�˵���ֽ�����к���CO�����Բ���立ֽ������![]() ��
��![]() ��CO��
��CO��![]() ����Ӧ�Ļ�ѧ����ʽΪ
����Ӧ�Ļ�ѧ����ʽΪ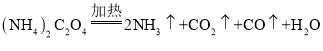 ��
��
��2����Ӧ��ʼǰ��ͨ�뵪����Ŀ�����ž�װ���ڵĿ���������CO�������ϼ��ȷ�����ը������ֹ�����е�![]() ����ʵ��,�ʴ�Ϊ���ž�װ���ڵĿ���������CO�������ϼ��ȷ�����ը������ֹ�����е�
����ʵ��,�ʴ�Ϊ���ž�װ���ڵĿ���������CO�������ϼ��ȷ�����ը������ֹ�����е�![]() ����ʵ�飻
����ʵ�飻
��3��װ��E��F����֤����立ֽ�����к���CO������Ҫ�ѷֽ������![]() ��ȥ�����װ��C�������ǣ�����
��ȥ�����װ��C�������ǣ�����![]() �������CO�ļ���������ţ��ʴ�Ϊ������
�������CO�ļ���������ţ��ʴ�Ϊ������![]() �������CO�ļ���������ţ�
�������CO�ļ���������ţ�
��4������立ֽ������![]() �л�ԭ�ԣ�һ��������Ҳ����CuO��Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ
�л�ԭ�ԣ�һ��������Ҳ����CuO��Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��
��![]() �����Ը��������Һ�ζ���������ӣ���������������ԭ��Ӧ����ɫ�ĸ��������Һ����ɫ���ζ����յ�ʱ��ʵ������Ϊ�����������һ��
�����Ը��������Һ�ζ���������ӣ���������������ԭ��Ӧ����ɫ�ĸ��������Һ����ɫ���ζ����յ�ʱ��ʵ������Ϊ�����������һ��![]() ��Һʱ����Һ����ɫ��Ϊdz��ɫ���Ұ�����ڲ���ɫ���������εζ��������Ը��������Һ�������֪����������һ������������̫�����������������ε�ƽ�����Ϊ
��Һʱ����Һ����ɫ��Ϊdz��ɫ���Ұ�����ڲ���ɫ���������εζ��������Ը��������Һ�������֪����������һ������������̫�����������������ε�ƽ�����Ϊ![]() ������������ԭ��Ӧ�еĵ����غ㼰Ԫ���غ㣬��
������������ԭ��Ӧ�еĵ����غ㼰Ԫ���غ㣬�� �����
�����![]() ������20mLѪҺ��Ʒ�к��еĸ�Ԫ�ص����ʵ���Ϊ
������20mLѪҺ��Ʒ�к��еĸ�Ԫ�ص����ʵ���Ϊ![]() ����
����![]() �����ѪҺ�и�Ԫ�صĺ���Ϊ
�����ѪҺ�и�Ԫ�صĺ���Ϊ![]() ���ʴ�Ϊ�����������һ��
���ʴ�Ϊ�����������һ��![]() ��Һʱ����Һ����ɫ��Ϊdz��ɫ���Ұ�����ڲ���ɫ��
��Һʱ����Һ����ɫ��Ϊdz��ɫ���Ұ�����ڲ���ɫ��![]() ��
��

 ��ĩ�����ϵ�д�
��ĩ�����ϵ�д�����Ŀ��Ϊ�����ú��������,�����Ȱ�úת��ΪCO��H2���ٽ�����ת��Ϊ�״���ijʵ����Ա��һ���¶���2L���ܱ������У�����һ������H2��CO��������Ӧ��2H2(g)+CO(g)![]() CH3OH(g)���ⶨ�IJ���ʵ���������£�
CH3OH(g)���ⶨ�IJ���ʵ���������£�
t/s | 0 | 500 | 1000 |
c(H2)/ mol.L-1 | 5.00 | 3.52 | 2.48 |
c(CO)/ mol.L-1 | 2.50 |
(1)��500s����H2��ʾ�Ļ�ѧ��Ӧ������________________��
(2)��1000s����CO��ʾ�Ļ�ѧ��Ӧ������________________��1000sʱCO��ת������________��
(3)��500sʱ���ɵļ״���Ũ����________
����Ŀ��CO��CO2�ǻ�ʯȼ��ȼ�պ����Ҫ���
��1�������������ڰ�װ�Ĵ�ת��������ʹ����β���е���Ҫ��Ⱦ��ת��Ϊ���Ĵ���ѭ�����ʡ���֪��N2(g)��O2(g)===2NO(g)��H=��180.5kJ��mol��1
2C(s)��O2(g)===2CO(g)��H=��221.0kJ��mol��1
C(s)��O2(g)===CO2(g)��H=��393.5kJ��mol��1
��Ӧ2NO(g)��2CO(g)===N2(g)��2CO2(g)����H=________kJ��mol��1��
��2����֪����ӦCO2(g)![]() CO(g)+O(g)���ܱ�������CO2�ֽ�ʵ��Ľ����ͼ1����Ӧ2CO2(g)
CO(g)+O(g)���ܱ�������CO2�ֽ�ʵ��Ľ����ͼ1����Ӧ2CO2(g)![]() 2CO(g)+O2(g)��1molCO2�ڲ�ͬ�¶��µ�ƽ��ֽ�����ͼ2��
2CO(g)+O2(g)��1molCO2�ڲ�ͬ�¶��µ�ƽ��ֽ�����ͼ2��
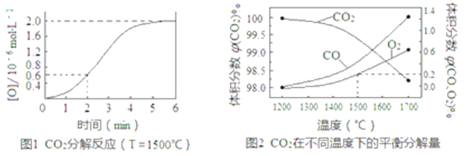
�ٷ���ͼ1����2min��v(CO2)=_______��5minʱ�ﵽƽ�⣬ƽ��ʱc��CO��=_______��
�ڷ���ͼ2��1500��ʱ��Ӧ��ƽ�⣬��ʱ�������Ϊ1L����Ӧ��ƽ�ⳣ��K=______(����������1λС��)��
��3��Ϊ̽����ͬ������CO��H2�ϳ�CH3OH��ѡ����Ч����ijʵ���ҿ���CO��H2�ij�ʼͶ�ϱ�Ϊ1��3����ʵ�飬�õ��������ݣ�
ѡ�� | T/K | ʱ��/min | �������� | �״��ĺ���(%) |
A | 450 | 10 | CuO-ZnO | 78 |
B | 450 | 10 | CuO-ZnO-ZrO2 | 88 |
C | 450 | 10 | ZnO-ZrO2 | 46 |
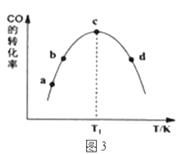
���ɱ�1��֪���÷�Ӧ����Ѵ���Ϊ____________�����ţ���ͼ3��a��b��c��d�ĵ��Ǹ��¶���CO��ƽ��ת���ʵ���____________��
�����������COת��ΪCH3OH��ƽ��ת���ʵĴ�ʩ��____________��
A��ʹ�ô���CuO��ZnO��ZrO2B���ʵ����ͷ�Ӧ�¶�
C������CO��H2�ij�ʼͶ�ϱ�D�������£��ٳ���amolCO��3amolH2
����Ŀ��ij�о���ѧϰС��ͬѧΪ��̽��������ͬ���¶Ⱥ�ѹǿ�£���ͬ������κ����嶼������ͬ��Ŀ�ķ������������Խ̲�����أ���ѧ̽����Ϊ�������������ͼʵ��װ�ò���¼���ʵ�����ݡ�
��ʵ��װ�ã�
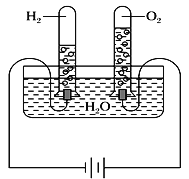
������ʵ�����ݣ�
�¶� | ѹǿ | ʱ�� | ˮ����H2O������ | H2��� | O2��� |
30 �� | 101 kPa | 0 | 300 g | 0 | 0 |
30 �� | 101 kPa | 4���� | 298.2 g | 1.243 L |
��ش��������⣺
(1)4����ʱH2��O2�����ʵ����ֱ���________mol��________mol��
(2)���¶��£�����Ħ�������__________��
(3)�ڸ�ʵ�������£�3 mol O2����������Ϊ____________L