��Ŀ����
����Ŀ��ͭ����Ͻ�����������ʹ�õĽ������ϡ�
��1����̬ͭԭ�ӵĵ����Ų�ʽΪ___________________��
��2��ͼ1��Cu2O�ľ�����Cuԭ����λ��Ϊ_________________��
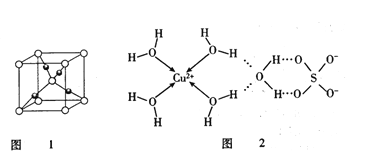
��3����ѧ��ͨ��X�����Ʋ���мȺ�����λ�����ֺ����������ṹʾ��ͼ�ɱ�ʾ��ͼ2��
��SO42-��Sԭ�ӵ��ӻ�����Ϊ________________��д��һ����SO42-��Ϊ�ȵ�����ķ��ӵĻ�ѧʽ____________________��
�������Ļ�ѧʽ����������ʽ�ɱ�ʾΪ_______________��1mol����������������ĿΪ��______________ ��
���𰸡�1s22s22p63s23p63d104s1 2 sp3 �ӻ� CCl4 [Cu(H2O)4] SO4��H2O 18 mol
��������
��1����̬ͭԭ�ӵĵ����Ų�ʽΪ: 1s22s22p63s23p63d104s1��
��2��ͼ1��Cu2O�ľ�����Cuԭ�Ӹ���Ϊ4����ԭ����Ϊ8![]() 1/8+1 =2, Cuԭ����λ��Ϊ��2��
1/8+1 =2, Cuԭ����λ��Ϊ��2��
���ɽṹʾ��ͼ֪S�γ���4������O�γ���3����������һ���¶Ե��ӣ�S.O�γ���sp3 �ӻ�������SO42-��Sԭ�ӵ��ӻ�����Ϊsp3 �ӻ���SO42-�۲���ӶԸ���=4+1/2![]() (6+2-4
(6+2-4![]() 2)=4,��ռ乹���������壬����CCl4��ԭ�����ͼ۵�������ȣ�������SO42-��Ϊ�ȵ�����ķ���ΪCCl4������𰸣�sp3 �ӻ� CCl4��
2)=4,��ռ乹���������壬����CCl4��ԭ�����ͼ۵�������ȣ�������SO42-��Ϊ�ȵ�����ķ���ΪCCl4������𰸣�sp3 �ӻ� CCl4��
���ھ���ṹ��һ��ͭ������4��ˮ�����γ���λ�������Ի�ѧʽΪ[Cu(H2O)4] SO4��H2O�������Ļ�ѧʽ����������ʽ�ɱ�ʾΪ[Cu(H2O)4] SO4��H2O
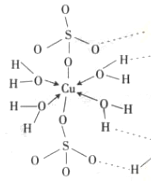
�ɵ�����ƽ��ṹʾ��ͼ��֪1mol����������������ĿΪΪ18mol��

����Ŀ�����������(Na2S2O3)����Ҫ�Ļ���ԭ�ϣ�������ˮ�������Ի���Ի������ȶ�����������Һ�зֽ����S��SO2��
��.Na2S2O3���Ʊ�����ҵ�Ͽ��÷�Ӧ��2Na2S+Na2CO3+4SO2=3Na2S2O3 +CO2�Ƶã�ʵ����ģ��ù�ҵ���̵�װ����ͼ��ʾ��
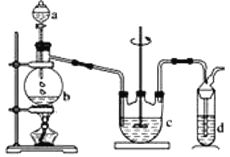
��1��b�з�Ӧ�����ӷ���ʽΪ____��c���Լ�Ϊ_____��
��2����Ӧ��ʼ��c�����л��Dz��������ֱ���塣�˻�������_____��
��3��ʵ����Ҫ����SO2�������ʣ����Բ�ȡ�Ĵ�ʩ��______(д������)��
��4��Ϊ�˱�֤��������ƵIJ�����ʵ����ͨ���SO2�����ܹ�����ԭ����____��
��5���Ʊ��õ���Na2S2O3�п��ܺ���Na2SO3��Na2SO4�����ʡ����ʵ�飬����Ʒ���Ƿ����Na2SO4��_____��
��.̽��Na2S2O3����������ӵ�������ԭ��Ӧ��
���ϣ�����Fe3++3S2O32-![]() Fe(S2O3)33-(�Ϻ�ɫ)
Fe(S2O3)33-(�Ϻ�ɫ)
����Ag2S2O3Ϊ��ɫ������Ag2S2O3�����ڹ�����S2O32-
װ�� | ��� | �Լ�X | ʵ������ |
| �� | Fe(NO3)3��Һ | ��Ϻ���Һ�ȱ���Ϻ�ɫ, 30s����Һ������Ϊ��ɫ |
�� | AgNO3��Һ | �����ɰ�ɫ��״�����������ܽ⣬�õ���ɫ��Һ |
��6������ʵ��ٵ��������ж�����Fe3+��S2O32-��ԭΪFe2+���ӻ�ѧ��Ӧ���ʺ�ƽ��ĽǶȽ���ʵ��������_____��
��7��ͬŨ�������ԣ�Ag+ > Fe3+��ʵ�����Ag+δ����������ԭ��Ӧ��ԭ����____��
��8����һ��̽��Ag+��S2O32-��Ӧ��
װ�� | ��� | �Լ�X | ʵ������ |
| �� | AgNO3��Һ | �����ɰ�ɫ��״�����������ܿ��Ϊ��ɫ����ɫ�����Ϊ��ɫ������ |
ʵ����а�ɫ��״��������Ϊ��ɫ����(Ag2S)�Ļ�ѧ����ʽ���£�������ʵ����ʺ�ϵ����Ag2S2O3+___=Ag2S+___
��9����������ʵ�飬Na2S2O3����������ӷ���������ԭ��Ӧ��___�й�(д������)��
����Ŀ����ͼ a��b��c��d��Ϊ���缫����ѡ��ĵ������Һ���±���Ҫ���������ǣ��ٹ���һ��ʱ��ײ۵��ҺpH���������Ҳ۵��ҺpH�½�����b��c�����ŵ����ӵ����ʵ�����ȡ���Ӧѡ�õĵ��Һ��
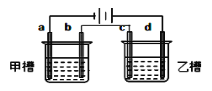
�� | A | B | C | D |
�ײ� | KCl | NaCl | NaOH | Cu(NO3)2 |
�Ҳ� | AgNO3 | NaNO3 | CuSO4 | NaCl |
A. A B. B C. C D. D