��Ŀ����
2��ʵ�����ù����ռ�����0.1mol/L��NaOH��Һ500mL���ش��������⣺��1��������ҪNaOH���������2.0g
��2����������������Ʒ������ʱ������ʹ�õ���������Ʒ�Т��ձ�����ҩ�ס���������ƽ����ȱ�ٵIJ��������Dz�������500mL����ƿ����ͷ�ι�
��3������ʱ��һ���Ϊ���¼������裺
�ٳ������ڼ��㡡���ܽ⡡��ҡ�� ����Һ ��ϴ�� �߶��ݡ���װƿ
����ȷ�IJ���˳��Ϊ���ڢ٢ۢݢޢߢܢࡡ������ţ�
��4�����ƹ����У���������������ȷ�����в�����������ƫ�ߵ��Ǣݣ�����ţ�
�ٶ���ҡ�Ⱥ�Һ���½����ּ�ˮ���̶���
��δϴ���ձ���������
������ƿ�����������������ˮ
�ܳ���NaOH��ʱ��̫��
�ݶ���ʱ���ӿ̶ȣ�
���� ��1������m=cVM������Ҫ�������Ƶ�������
��2����������һ�����ʵ���Ũ����Һ��һ�㲽��ѡ��������
��3����������һ�����ʵ���Ũ����Һ��һ�㲽������
��4������C=$\frac{n}{V}$�ж�������Һ�������nƫС��Cƫ����������Һ��Ũ��ƫ�ͣ����nƫ���VƫС����������Һ��Ũ��ƫ�ߣ�
��� �⣺��1������0.1mol/L��NaOH��Һ500mL����Ҫ�������ƹ��������m=0.5L��0.1mol/L��40g/mol=2.0g���ʴ�Ϊ��2.0��
��2������һ�����ʵ���Ũ����Һ��һ�㲽�裺�������ܽ⡢��ȴ��ת�ơ�ϴ�ӡ�ת�ơ����ݡ�ҡ�ȣ��õ��������У��ձ���ҩ�ס�������ƽ����������500mL����ƿ����ͷ�ιܣ�
�ʴ�Ϊ����������500mL����ƿ����ͷ�ιܣ�
��3������һ�����ʵ���Ũ����Һ��һ�㲽�裺�������ܽ⡢��ȴ��ת�ơ�ϴ�ӡ�ת�ơ����ݡ�ҡ�ȣ������������Ǣڢ٢ۢݢޢߢܢࣻ
�ʴ�Ϊ���ڢ٢ۢݢޢߢܢࣻ
��4���ٶ���ҡ�Ⱥ�Һ���½��������ģ��ּ�ˮ���̶��ᵼ����ҺŨ��ƫ�ͣ��ʢٴ���
��δϴ���ձ���������������������ʧ�����ʵ���ƫС����Һ��Ũ��ƫ�ͣ��ʢڴ���
������ƿ�����������������ˮ�������ʵ����ʵ�������Һ������������Ӱ�죬�ʢ۴���
�ܳ���NaOH��ʱ��̫�������������׳��⣬��ȡ���������Ƶ�����ƫС�����ʵ����ʵ���ƫС����Һ��Ũ��ƫ�ͣ��ʢܴ���
�ݶ���ʱ���ӿ̶ȣ�������Һ�����ƫС����Һ��Ũ��ƫ�ߣ��ʢ���ȷ��
��ѡ�ݣ�
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƣ��ѶȲ�����ȷ�Ʊ�ԭ���ǽ���ؼ���ע���������ķ����ͼ��ɣ�

 ��ѧ����ϵ�д�
��ѧ����ϵ�д� �ο�������ϵ�д�
�ο�������ϵ�д�| A�� | ̼��Ƶμ����CO32-+2H+�TCO2+H2 O | |
| B�� | �������������ᷴӦ��H++OH-�TH2O | |
| C�� | ������ͭ�����ᷴӦCu��OH��2+2H+�TCu2++2H2O | |
| D�� | ��Ƭ�������ṯ��Һ�У�Al+Hg2+�TAl3++Hg |
| A�� | �����г����Ĺ����β����в�����ˮ�ࡢ�մ� | |
| B�� | �����մɵ���Ҫԭ������� | |
| C�� | ��ͨ��������Ҫ�ɷ�ֻ��SiO2 | |
| D�� | ������ˮ����ʯ��ʯ�����Ϊ��Ҫԭ������ |
�ټȲ�����ˮ�ֲ������� �ڶ������нϴ��� �۲��ױ�X�����������������Ƽ��������ã�
| A�� | ֻ�Т� | B�� | �ٺ͢� | C�� | �ٺ͢� | D�� | �ں͢� |
| A�� | ������Ӧ | B�� | ȡ����Ӧ | C�� | �ӳɷ�Ӧ | D�� | ��ȥ��Ӧ |
| A�� | �٢ܢڢ� | B�� | �ܢۢڢ� | C�� | �ڢۢܢ� | D�� | �٢ܢۢ� |
| A�� | NaCl����ˮ�ڵ��������µ���������Ӻ������� | |
| B�� | ����ʱ���ɵ���������H+ �Ļ���������� | |
| C�� | CO2 ��ˮ��Һ�ܵ��磬����CO2 �ǵ���� | |
| D�� | ����NaCl�����磬��NaCl�ǵ���� |
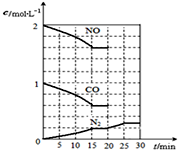 ���Ŵ�����Ⱦ���������أ��������ڡ�ʮ���塱�ڼ䣬����������SO2���ŷ�������8%���������NOx���ŷ�������10%��Ŀǰ������������Ⱦ�ж��ַ�����
���Ŵ�����Ⱦ���������أ��������ڡ�ʮ���塱�ڼ䣬����������SO2���ŷ�������8%���������NOx���ŷ�������10%��Ŀǰ������������Ⱦ�ж��ַ�����