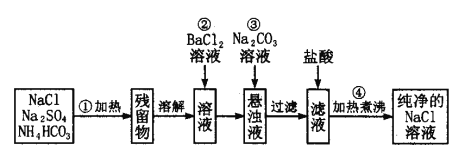
题目内容
【题目】Ⅰ.(1)将CO2转化为甲醇可以有效利用资源,同时又可控制温室气体,原理为:CO2+3H2![]() CH3OH+H2O
CH3OH+H2O
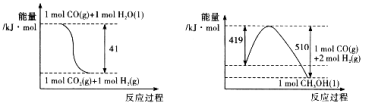
由下图所示的能量变化,写出将CO2转化为甲醇的热化学方程式:
Ⅱ.回收铅蓄电池的电极填充物(铅膏,主要含PbO、PbO2、PbSO4),可制备热稳定剂三盐基硫酸铅(组成可表示为3PbO·PbSO4·H2O),其实验流程如下:

(2)物质X可以循环利用,该物质是。最后一次过滤之后需要洗涤,检验三盐基硫酸铅是否洗净的方法是 ;
(3)流程中不直接利用H2SO4溶液与PbO、PbCO3反应制取PbSO4,原因可能是:____________;
(4)生成三盐基硫酸铅的离子反应方程式为 ;
(5)向铅膏浆液中加入Na2SO3溶液的目的是将其中的PbO2还原为PbO。若实验中所取铅膏的质量为47.8g,其中PbO2的质量分数为15.0%,则要将PbO2全部还原,至少需加____ mL1.0mol/LNa2SO3溶液。
【答案】
Ⅰ.(1)CO2(g)+3H2(g)===CH3OH(l)+H2O(l)ΔH=-50kJ/mol
Ⅱ.(2)HNO3溶液;取最后一次洗涤液,加入足量稀盐酸,再加氯化钡溶液,观察是否产生白色沉淀,若无沉淀,则已洗净(其他合理答案也可)
(3)PbSO4不溶于水,覆盖在固体表面阻碍反应的进一步发生(其他合理答案也可)
(4)4PbSO4+6OH-===3PbO·PbSO4·H2O+3SO+2H2O
(5)30;
【解析】
试题分析:(1)根据图1中能量变化,可得热化学方程式为:①CO(g)+H2O(l)=CO2(g)+H2(g)△H=-41kJ/mol,②CO(g)+2H2(g)=CH3OH(l)△H=-(510-419)kJ/mol=-91KJ/mol,根据盖斯定律,②-①可得:CO2(g)+3H2(g)=CH3OH(l)+H2O(l)△H=-50KJ/mol;故答案为:CO2(g)+3H2(g)=CH3OH(l)+H2O(l)△H=-50KJ/mol;
(2)分析流程可知,PbO和PbCO3在硝酸的作用下转化成Pb(NO3).Pb(NO3)中加稀H2SO4转化成PbSO4和硝酸,因此X为HNO3,可循环利用,检验硫酸根离子的方法为:取少量晶体溶于蒸馏水,然后用盐酸酸化,再滴BaCl2溶液,若出现白色沉淀,即证明该晶体中含有SO42-,故答案为:HNO3溶液;取少量溶液,加入足量稀盐酸,再加氯化钡溶液,观察是否产生白色沉淀;
(3)生成的硫酸铅难溶于水,覆盖在固体PbO、PbCO3的表面,阻碍反应的进一步发生,故答案为:PbSO4不溶于水,覆盖在固体表面阻碍反应的进一步发生;
(4)从流程看,硫酸铅和氢氧化钠反应生成三盐基硫酸铅和硫酸钠,反应方程式为:4PbSO4+6NaOH=3PbOPbSO4H2O+3Na2SO4+2H2O,离子方程式为:4PbSO4+6OH-=3PbOPbSO4H2O+3SO42-+2H2O,故答案为:4PbSO4+6OH-=3PbOPbSO4H2O+3SO42-+2H2O;
(5)氧化铅的物质的量为:![]() =0.03mol,
=0.03mol,
PbO2~Na2SO3
1mol1mol
0.03moln
n=0.03mol,V=![]() =0.03L=30mL,故答案为:30。
=0.03L=30mL,故答案为:30。