��Ŀ����
10��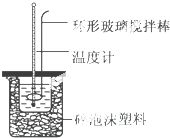 ʵ������50mL 0.50mol•L-1���ᡢ50mL 0.55mol•L-1 NaOH��Һ����ͼ��ʾװ�ã����вⶨ�к��ȵ�ʵ�飬�õ��±��е�����
ʵ������50mL 0.50mol•L-1���ᡢ50mL 0.55mol•L-1 NaOH��Һ����ͼ��ʾװ�ã����вⶨ�к��ȵ�ʵ�飬�õ��±��е�����| ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�� ��t2/�� | |
| ���� | NaOH��Һ | ||
| 1 | 20.2 | 20.3 | 23.7 |
| 2 | 20.3 | 20.5 | 23.8 |
| 3 | 21.5 | 21.6 | 24.9 |
��1��ʵ��ʱ������ͭ˿��������滷�β����������������Cu���ȿ죬������ʧ��
��2���ڲ�����ȷ��ǰ���£�����к��Ȳⶨȷ�ԵĹؼ������װ�õı���Ч����
��3�������ϱ����������ݽ��м��㣬���ʵ���õ��к��ȡ�H=-56.8 kJ/mol[�����NaOH��Һ���ܶȰ�1g•cm-3���㣬��Ӧ������Һ�ı����ݣ�c����4.18J•��g•�棩-1����]�������0.5mol/L��������NaOH�������ʵ�飬��ʵ���в�õġ��к��ȡ���ֵ��ƫ���ƫ����ƫС�����䡱����
��4����ijͬѧ��������װ����ʵ�飬��Щ�������淶����ɲ���к��ȵ���ֵƫ�ͣ�����������ܵ�ԭ����ABE��
A������������¶Ⱥ��¶ȼ�û����ˮ��ϴ�ɾ�
B������Ͳ�е�����������Һ����С�ձ�ʱ�����ٻ�
C������ʵ��ĵ������½ϸ�
D������ȡ����ʱ���Ӷ���
E�����ձ��ĸǰ��м�С��̫��
���� ��1���������ȿ죬������ʧ�ࣻ
��2���к��Ȳⶨʵ��ɰܵĹؼ��DZ��¹�����
��3����������η�Ӧ���¶Ȳ���ݹ�ʽQ=cm��T���������0.05mol��ˮ�ų��������������к��ȵĸ��������Ӧ�ȣ��������ƹ�������ˮ���ȣ�
��4������ʵ��Ĺؼ��DZ��£����װ��������ɢʧ����ᵼ�½��ƫ�ͣ�����ʵ�����õ����Լ��Լ�ʵ�����֪ʶ���жϣ�
��� �⣺��1�����ܽ����β����������Ϊͭ˿���������Ϊͭ˿��������ȵ������壬
�ʴ�Ϊ��Cu���ȿ죬������ʧ��
��2���к��Ȳⶨʵ����ҪĿ���Dz�����Ӧ�ų����������٣�����ʵ��ɰܵĹؼ��DZ��¹�������������к��Ȳⶨȷ�ԵĹؼ������װ�õı���Ч����
�ʴ�Ϊ�����װ�õı���Ч����
��3����2����1��ʵ�������NaOH��Һ��ʼƽ���¶�Ϊ20.25�棬��Ӧǰ���¶Ȳ�Ϊ��3.45�棻
��2��ʵ�������NaOH��Һ��ʼƽ���¶�Ϊ20.40�棬��Ӧǰ���¶Ȳ�Ϊ��3.40�棻
��3��ʵ�������NaOH��Һ��ʼƽ���¶�Ϊ21.55�棬��Ӧǰ���¶Ȳ�Ϊ��3.35�棻
50mL 0.50mol•L-1���ᡢ50mL 0.55mol•L-1 NaOH��������Ϊm=100mL��1g/mL=100g��c=4.18J/��g•�棩��
���빫ʽQ=cm��T������0.025mol��ˮ�ų�����Q=4.18J/��g•�棩��100g��$\frac{3.45��+3.40��+3.35��}{3}$=1421.2J=1.4212KJ��
������0.025mol��ˮ�ų�����1.4212KJ����������1mol��ˮ�ų�����Ϊ$\frac{1.4212KJ��1mol}{0.025mol}$=56.8kJ������ʵ���õ��к��ȡ�H=-56.8kJ/mol��
�������ƹ�������ˮ���ȣ�����ʵ���в�õġ��к��ȡ���ֵ��ƫ��
�ʴ�Ϊ��-56.8 kJ/mol��ƫ��
��4��A������������¶Ⱥ��¶ȼ�û����ˮ��ϴ�ɾ����ڲ����¶�ʱ���ᷢ����ͼ���кͣ��¶ȼ�ʾ���仯ֵ��С�����Ե���ʵ�����к��ȵ���ֵƫС����A��ȷ��
B������Ͳ�е�����������Һ����С�ձ�ʱ�����ٻ����ᵼ��һ����������ɢʧ��ʵ�����к��ȵ���ֵƫС����B��ȷ��
C������ʵ������ºͷ�Ӧ�ȵ�����֮���أ���C����
D������ȡ����ʱ���Ӽ�������ʹ��ʵ����ȡ���������Ҫ�������������������Ա�֤��ȫ��Ӧ����ʹ���кͺ��ȵIJⶨ����ƫ�ߣ���D����
E�����ձ��ĸǰ��м�С��̫�ᵼ��һ��������ɢʧ�����Բ����ֵ���ͣ���E��ȷ��
��ABE��ȷ��
�ʴ�Ϊ��ABE��
���� ���⿼���к��ȵIJⶨ����Ŀ�Ѷ��еȣ�ע���������㹫ʽ��Ӧ����c=4.18J/��g•�棩��Ҫע��������λ�Ļ��㣬�����ڿ���ѧ����ʵ�������ͼ���������

 ��ʦ������Ԫ��ĩ���100��ϵ�д�
��ʦ������Ԫ��ĩ���100��ϵ�д� ��У������Ԫͬ��ѵ��������ϵ�д�
��У������Ԫͬ��ѵ��������ϵ�д�| A�� | NH3 | B�� | H2S | C�� | SO2 | D�� | HCl |
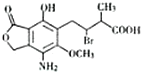 ij�л���M�Ľṹ��ʽ��ͼ��ʾ���������ʵ�����M��һ�������·ֱ�������ơ�����������Һ��̼��������Һ��Ӧ�������ĵ��ơ��������ơ�̼�����Ƶ����ʵ���֮��Ϊ��������
ij�л���M�Ľṹ��ʽ��ͼ��ʾ���������ʵ�����M��һ�������·ֱ�������ơ�����������Һ��̼��������Һ��Ӧ�������ĵ��ơ��������ơ�̼�����Ƶ����ʵ���֮��Ϊ��������| A�� | 1��1��1 | B�� | 2��4��1 | C�� | 1��2��1 | D�� | 1��2��2 |
| ѡ�� | �������ʵ | ���� |
| A | ���ǡ����ۡ���ά�ص�����ζ | �����ڣ������ڵ���ø���������ܷ���ˮ�⣬���������� |
| B | úͨ��������Һ���Ȼ�ѧ�仯������Ч�ؿ������� | úͨ��������Һ�����������Ʒ����ĺ�������� |
| C | �ع��;��������ӹ���������ɻ�������ȼ�� | �ع��͵ijɷ���Һ̬�� |
| D | �ڸ�����Ʒ�ı������һ����������Ч��ֹ�䱻��ʴ | �γ�ԭ���ʱ���Ǹ�������������������Ʒ��Ϊ���������� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| ���� | ���� |
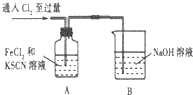 | 1��A����Һ��� 2���Ժ�A����Һ�ɺ�ɫ��Ϊ��ɫ |
��2��B�з�Ӧ�����ӷ���ʽ��Cl2+2OH-�TCl-+ClO-+H2O��
��3��Ϊ̽������2������ԭ��С���������ʵ�飺
��ȡA�л�ɫ��Һ�������Թ��У�����NaOH��Һ���к��ɫ�������ɣ���ԭ��Һ��һ������Fe3+��
��ȡA�л�ɫ��Һ�������Թ��У����������KSCN��Һ�����յõ���ɫ��Һ����С��ͬѧ�˵ó����ۣ���������2��ԭ����SCN-��Cl2�����˷�Ӧ��
��4����С��ͬѧ����SCN-���ܱ�Cl2�����ˣ������ֽ���������̽����
��ȡA�л�ɫ��Һ���Թ��У������������ữ��BaCl2��Һ��������ɫ�������ɴ�֤��SCN-�б�������Ԫ����SԪ�أ�
����ʵ����SCN-�е�Ԫ�ر�����ΪNO3-������NO3-���ڵķ�����ȡ����ͭ�����Թ��У�����A�л�ɫ��Һ��һ������ϡ���ᣬ���ȣ��۲쵽�Թ��Ϸ��к���ɫ�������ɣ���֤��SCN-�е�Ԫ�ر�����ΪNO3-��
����֪SCN-�и�ԭ�Ӿ�����8�����ȶ��ṹ����д��SCN-�ĵ���ʽ
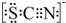 ��
����5����SCN-��Cl2��Ӧ����1molCO2����ת�Ƶĵ�������16mol��
| A�� | Fe2+ | B�� | Fe3+ | C�� | Fe3+��Cu2+ | D�� | Fe2+ Cu2+ |
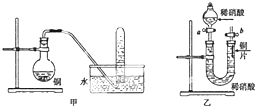 ��ͼ�Ǽס�����λͬѧ̽��ͭ��ϡ���ᣮ��Ӧ��ԭ�����ʵ��װ��ͼ����ش��������⣺
��ͼ�Ǽס�����λͬѧ̽��ͭ��ϡ���ᣮ��Ӧ��ԭ�����ʵ��װ��ͼ����ش��������⣺