��Ŀ����
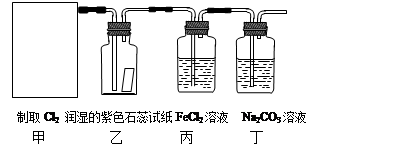
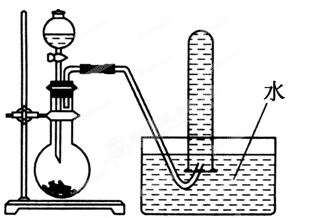
��1����ͼ��ʾ������������ͨ��ʢ�и�����ɫ�����Ĺ��ƿ��ʢ�г�ʪ��ɫ�����Ĺ��ƿ���ɹ۲쵽��������___ ___________��

��2��Ϊ��ֹ����β����Ⱦ������������ˮ�����Ե����ʣ� ���� ��Һ���ն����������ԭ���ǣ��û�ѧ����ʽ��ʾ��_________��������һԭ������ҵ�ϳ������۵�ʯ�������չ�ҵ����β���Ƶ�Ư�ۣ�Ư�۵���Ч�ɷ���__ ____���ѧʽ��
��3���ݡ���������������2004��4��15����16�գ�λ�������н���������ԭ�����ܳ����
��������й©�ͱ�ը�¹ʡ��������߷���ʱ������9�����¹���ʧ��������3�����ˣ�15���˱�������ɢ�����������뱬ը�ֳ�ʱ�������ý���һ��Ũ�ȵ�ij�����ʵ�ˮ��Һ��ë����ס���ӣ������˲��õĸ������� ��

��2��Ϊ��ֹ����β����Ⱦ������������ˮ�����Ե����ʣ� ���� ��Һ���ն����������ԭ���ǣ��û�ѧ����ʽ��ʾ��_________��������һԭ������ҵ�ϳ������۵�ʯ�������չ�ҵ����β���Ƶ�Ư�ۣ�Ư�۵���Ч�ɷ���__ ____���ѧʽ��
��3���ݡ���������������2004��4��15����16�գ�λ�������н���������ԭ�����ܳ����
��������й©�ͱ�ը�¹ʡ��������߷���ʱ������9�����¹���ʧ��������3�����ˣ�15���˱�������ɢ�����������뱬ը�ֳ�ʱ�������ý���һ��Ũ�ȵ�ij�����ʵ�ˮ��Һ��ë����ס���ӣ������˲��õĸ������� ��
| A��NaOH | B��NaCl | C��KBr | D��Na2CO3 |
��6�֣���1����ʪ��ɫ������ɫ��������ɫ��������ɫ 1��
��2���������ƣ�NaOH����1��;2NaOH+Cl2=NaClO+NaCl+H2O 2�֣�Ca(ClO)2 1�� ��3��D 1��
��2���������ƣ�NaOH����1��;2NaOH+Cl2=NaClO+NaCl+H2O 2�֣�Ca(ClO)2 1�� ��3��D 1��
�����������1�����������Ư���ԣ����Գ�ʪ��ɫ������ɫ��������ɫ��������ɫ��
��2����ˮ�����ԣ����Կ���������������Һ���ն������������Ӧ�Ļ�ѧ����ʽ��2NaOH+Cl2��NaClO+NaCl+H2O��Ư�����ڻ�������Ч�ɷ��Ǵ�����ơ�
��3���������ƾ��и�ʴ�ԣ�A����ȷ���Ȼ��Ʋ��������������廯�������������������˵����壬�������ж���������ȷ�Ĵ�ѡD��
�������������е��Ѷȵ����⣬����ע�ػ��������ؿ���ѧ��������������������ѧ������˼ά�����ͷ�ɢ˼ά������Ҳ����������ѧ���Ĺ淶��������������Ĺؼ���������ס�������ʵĻ�ѧ���ʡ�

��ϰ��ϵ�д�
 ���������ν�ϵ�д�
���������ν�ϵ�д�
�����Ŀ
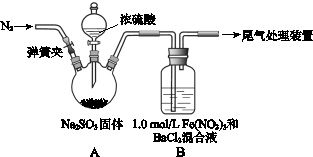
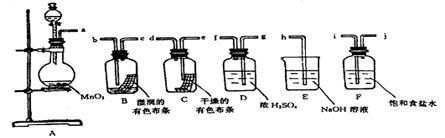
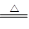 MnCl2 + Cl2��+ 2H2O��ijѧϰС�����ô�ԭ�������ͼ��ʾװ����ȡ������̽�������ʡ�
MnCl2 + Cl2��+ 2H2O��ijѧϰС�����ô�ԭ�������ͼ��ʾװ����ȡ������̽�������ʡ�