��Ŀ����
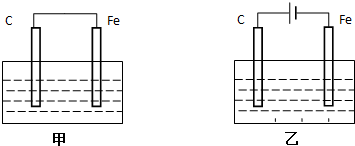
8����ͼ��ʾ����A�г���1mol X��1mol Y����B�г���2mol X��2mol Y����ʼʱVA=VB=aL������ͬ�¶Ⱥ��д������ڵ������£����������Է������з�Ӧ��X+Y?2Z+W����H��0 �������ʾ�Ϊ���壩���ﵽƽ��ʱ��VA=1.2aL���Իش�
��1��A�е�X��ת����aA=40%��
��2��A��B��X��ת����aA��aB�����������������=����
��3����K��һ��ʱ���ִﵽƽ��ʱ��A�����Ϊ2.6aL����ͨ���е�����������ƣ�
���� ����ͼ��֪��A���ֺ�ѹ��B���ֺ��ݣ�
��1���ȸ�����ͬ�����£����������֮�ȵ������ʵ���֮�ȼ���ƽ�����������ʵ������ٽ�Ϸ���ʽ����X��Ӧ�����ʵ�����������ת���ʹ�ʽ����X��ת���ʣ�
��2�����Bװ����ѹǿ�Ի�ѧƽ���Ӱ���ж�X��ת���ʴ�С��
��3�����ݵ��¡���ѹ�����£���������֮�ȵ������ʵ���֮�ȣ���֪A�г���1molX��1molY�����Ϊ1.2aL�����K���൱�ڳ�����3molX��3molY������AB�����Ϊ3.6aL���Ӷ��ó�A�������
��� �⣺��1����ѹ�����������£���������֮�ȵ�����������ʵ���֮�ȣ�2mol����ʱ�����ΪaL���ﵽƽ��ʱ��VA=1.2a L������ƽ�������������ʵ�����2.4mol��
��A���ʷ�Ӧ��mmol��
X��g��+Y��g��?2Z��g��+W��g�� ���ʵ�������
1mol 1mol
nmol ��2.4-2��mol
n=0.4
����X���ʵ�ת����=$\frac{0.4mol}{1mol}$��100%=40%��
�ʴ�Ϊ��40%��
��2��Bװ�����ڵ��������£���Ӧ������Ӧ�����ƶ�ʱ�������������ʵ�������������������ѹǿ��������ѹǿ������X��ת���ʣ�����X��ת���ʼ�С������B������X��ת���ʱ�AС��
�ʴ�Ϊ������
��3������Kʱ������װ�����ڵ��¡���ѹ�����·�Ӧ��ʹ����װ���������ѹǿ��Aװ���еij�ʼѹǿ��ͬʱ����A�г���1molX��1molY�ﵽƽ��ʱ��VA=1.2aL������Kʱ��AB�����干Ϊ3molX��3molY����������װ�����ڵ��¡���ѹ�����µķ�Ӧ���ﵽƽ��״̬ʱ���������������3.6aL������B��aL������A��2.6aL��
�ʴ�Ϊ��2.6a��
���� ���⿼���˻�ѧƽ����йؼ��㡢��ѧƽ��Ӱ�������ȣ��Ѷ��еȣ�ȷ����K������ƽ���뿪ʼA�е�ƽ��Ϊ��Чƽ���ǽ���ؼ���

| A�� | �����Ȼ�����Һ���ɣ����Ƶ���ˮ���Ȼ��� | |
| B�� | ������ˮ������Al��OH��3���壬��������ˮ����˵��Al��OH��3����Ư���� | |
| C�� | ��1molKOH����Һ��1molCO2��ȫ��Ӧ����Һ��c��K+��=c��HCO3-�� | |
| D�� | ��CH3COONa��Һ�м�������CH3COOH����ʹc��Na+��=c��CH3COO-�� |
| A�� | 12�� | B�� | 10�� | C�� | 8�� | D�� | 7�� |
| ���� | CH3OH | CH3OCH3 | H2O |
| c/��mol•L-1�� | 0.8 | 1.24 | 1.24 |
| A�� | ƽ��������¶ȣ�ƽ�ⳣ����400 | |
| B�� | ƽ��ʱ��c��CH3OCH3��=1.6 mol•L-1 | |
| C�� | ƽ��ʱ����Ӧ����������������40 kJ | |
| D�� | ƽ��ʱ���ټ�������ʼ������CH3OH������ƽ���CH3OHת�������� |
| t/s | 0 | 50 | 150 | 250 | 350 |
| n��PCl3��mol | 0 | 0.16 | 0.19 | 0.20 | 0.20 |
| A�� | ��Ӧ�� ǰ50s��ƽ������v��PCl5��=0.0032mol��L-1��s-1 | |
| B�� | ���������������䣬�����¶ȣ�ƽ��ʱc��PCl5��=0.11mol��L-1����÷�Ӧ�ġ�H��0 | |
| C�� | ��ͬ�¶��£���ʼʱ�������г���1.0molPCl5��0.20molPCl3��0.20molCl2��Ӧ�ﵽƽ��ǰv����v�� | |
| D�� | ��ͬ�¶��£���ʼʱ�������г���2.0molPCl3��2.0molCl2���ﵽƽ��ʱ��PCl3��ת����С��80% |
 ת��Ϊ
ת��Ϊ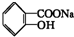 �ķ����ǣ�������
�ķ����ǣ�������| A�� | ������NaOH��Һ���Ⱥ��ټ����� | |
| B�� | ��Һ���ȣ�ͨ��������SO2 | |
| C�� | ��ϡ���Ṳ�Ⱥ�������NaOH��Һ | |
| D�� | ������ϡ���Ṳ�Ⱥ�������NaHCO3��Һ |