��Ŀ����
��19�֣�
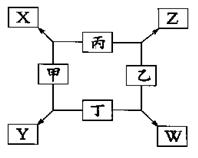
�й�Ԫ��X��Y��Z��W����Ϣ����
��ش��������⣺
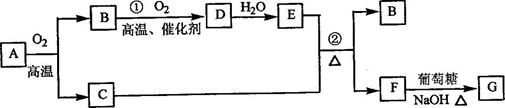
��1��W�ĵ�����Y������������Ӧ��ˮ���ﷴӦ�����ӷ���ʽΪ ��
ͭƬ��̼����ZX3��Һ���ԭ��أ�д�����������ĵ缫��Ӧ����ʽ
_______________________________________________��
��2������۵⻯����Һ�еμӼ���ZX3��Ũ��Һ������Ϊ_____________________����ط�Ӧ�����ӷ���ʽΪ_______________________________��
��3������������WX3��ҺΪԭ����ȡ��ˮWX3�����Ƶ�WX3��6H2O���壬��Ҫ��������__________________________����_____________________�����¼���WX3��6H2O���壬�ܽ���4��һ����ȡ��ˮWX3����ԭ���ǣ�������ӷ���ʽ��Ҫ˵����_______________________________��
�й�Ԫ��X��Y��Z��W����Ϣ����
| Ԫ�� | �й���Ϣ |
| X | ��������������������������֮��Ϊ4 |
| Y | ����������Ӧ��ˮ����ܵ������������ȵ����������� |
| Z | �����������г�������������Ʒ�ڳ�ʪ�������ױ���ʴ���� |
| W | �ؿ��к�����ߵĽ���Ԫ�� |
��1��W�ĵ�����Y������������Ӧ��ˮ���ﷴӦ�����ӷ���ʽΪ ��
ͭƬ��̼����ZX3��Һ���ԭ��أ�д�����������ĵ缫��Ӧ����ʽ
_______________________________________________��
��2������۵⻯����Һ�еμӼ���ZX3��Ũ��Һ������Ϊ_____________________����ط�Ӧ�����ӷ���ʽΪ_______________________________��
��3������������WX3��ҺΪԭ����ȡ��ˮWX3�����Ƶ�WX3��6H2O���壬��Ҫ��������__________________________����_____________________�����¼���WX3��6H2O���壬�ܽ���4��һ����ȡ��ˮWX3����ԭ���ǣ�������ӷ���ʽ��Ҫ˵����_______________________________��
��1��2Al + 2OH- + 2H2O = 2AlO2- + 3H2�� ��3�֣�
��2��2Fe3+ + 2e-
 2Fe2+ (��Fe3+ + e-
2Fe2+ (��Fe3+ + e-  Fe2+) ��3�֣�
Fe2+) ��3�֣���3����2�֣���Һ��Ϊ��ɫ ��2�֣� 2Fe3+ + 2I- = 2Fe2+ + I2 ��3�֣�
��4������Ũ������ȴ�ᾧ������ ��3�֣� �����HCl������2�֣�
Al3+ + 3H2O
 Al(OH)3 + 3H+���ڸ����HCl�����У�����AlCl3��ˮ�⣬��
Al(OH)3 + 3H+���ڸ����HCl�����У�����AlCl3��ˮ�⣬������AlCl36H2O�������Ȳ�����ˮ�������ܵõ�AlCl3�� ��3�֣�
��

��ϰ��ϵ�д�
 ��ʦָ����ĩ��̾�ϵ�д�
��ʦָ����ĩ��̾�ϵ�д� �����ܿ����ϵ�д�
�����ܿ����ϵ�д�
�����Ŀ