��Ŀ����
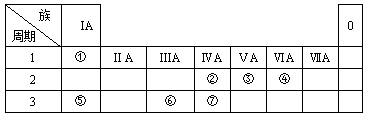
(12��)��ͼ��ʾ����֪��


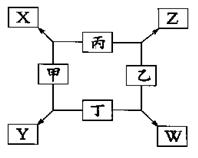
�ټס��ҡ�����Ϊǰ������Ԫ�ص����嵥�ʣ���Ϊ���嵥�ʡ�
����һ�������¼���������붡������������l��3���X��Y���ڲ�����Ԫ�ؼ׳ʸ��ۡ�
����һ������������������붡�������ʵ���֮��1��2��Ӧ���ֱ�����Z��W���ڲ�����Ԫ���ҳʸ��ۡ�
����գ�


(1)����_______________________������_______________________��
(2)д��X��Y�ĵ���ʽ
________________________________________��_________________________________��
(3)���붡��Ӧ����W�Ļ�ѧ����ʽ��
________________________________________________________________��
(4)�������Ӧ����X�Ļ�ѧ����ʽ��
________________________________________________________________��
(5)ʵ������ȡX�Ļ�ѧ����ʽ��
________________________________________________________________��




�ټס��ҡ�����Ϊǰ������Ԫ�ص����嵥�ʣ���Ϊ���嵥�ʡ�
����һ�������¼���������붡������������l��3���X��Y���ڲ�����Ԫ�ؼ׳ʸ��ۡ�
����һ������������������붡�������ʵ���֮��1��2��Ӧ���ֱ�����Z��W���ڲ�����Ԫ���ҳʸ��ۡ�
����գ�



(1)����_______________________������_______________________��
(2)д��X��Y�ĵ���ʽ
________________________________________��_________________________________��
(3)���붡��Ӧ����W�Ļ�ѧ����ʽ��
________________________________________________________________��
(4)�������Ӧ����X�Ļ�ѧ����ʽ��
________________________________________________________________��
(5)ʵ������ȡX�Ļ�ѧ����ʽ��
________________________________________________________________��
(1) N2 (1��) O2 (1��)
(2) �� (��2��)(3)O2 + 2Mg ="= " 2MgO (2��)
��4��N2 + 3H2 ="==" 2NH3(2��)
��5��2NH4Cl + Ca(OH)2 ="==" 2NH3��+ CaCl2 + 2H2O (2��)
|
|
|
��

��ϰ��ϵ�д�
�����Ŀ
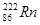 ����ԭ�ӵ���������
����ԭ�ӵ���������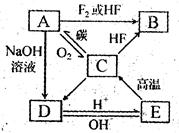
 B ��D ��E ��
B ��D ��E ��