��Ŀ����
��֪A��B��C��D��Ϊ������Ԫ�أ����ǵ�ԭ���������ε�����A�������Ϊһ�����ӵķǽ���Ԫ�أ�Cԭ�ӵ������������Ǵ�����3����C��D���γ����ֹ�̬���������һ��Ϊ����ɫ���壻B��C���γɶ�����̬�������A��B��C����Ԫ�ؿ��γ����Ӿ��壬�þ����и�Ԫ��ԭ�ӵ����ʵ���֮��ΪA��B��C=4��2��3��������������⣺��1������B��ԭ�ӽṹʾ��ͼ______________��д��C��D�γɵĵ���ɫ���廯����ĵ���ʽ______________��
��2��Ԫ��ԭ�ӵ����ʵ���֮��ΪA��B��C=4��2��3�ľ�������Ϊ______________����ˮ��Һ��______________�ԣ��䷴Ӧ�����ӷ���ʽΪ_______________________________��
��3����д����A2C��BA3�����е�������ͬ��������A��B��CԪ����������Ԫ����ɵ����ӷ��ţ���������______________��
��4��д����B��CԪ�������Ԫ��ԭ��������ΪB��C=7��12�Ļ�����Ļ�ѧʽ_______��
![]()
![]()
(2)����� �� ![]() +H2O
+H2O![]() NH3��H2O+H+
NH3��H2O+H+
(3)![]() ��
��![]() ��OH-��H3O+��
��OH-��H3O+��
��4��N2O3
�������������⣬�����Ƴ�A��H��C��OԪ�أ�DΪNaԪ�أ���ΪB��OԪ�ؿ��γɶ�����̬�����ԭ������B��O,H��B��O��Ԫ�ؿ��γ����Ӿ��壬����BԪ��ֻ����N��N��O��Ԫ����ɵ�Ԫ��ԭ��������Ϊ7��12����N��O��Ԫ�ص�ԭ�Ӹ�����Ϊ![]() =2��3���ʸû�����Ļ�ѧʽΪN2O3��
=2��3���ʸû�����Ļ�ѧʽΪN2O3��

��ϰ��ϵ�д�
 ����ͼ���������������ϵ�д�
����ͼ���������������ϵ�д� ����ѧҵ���Ե�����ϵ�д�
����ѧҵ���Ե�����ϵ�д�
�����Ŀ




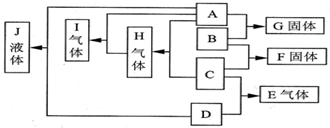

 ��֪A��B��C��D��Ϊ���壬E��F��Ϊ�����³ʹ�������ӻ����GΪ�Ȼ��ƣ�A��B��ȼ�յĻ���ʲ�ɫ����Ӧ���������������д������̣�����֮���ת����ϵ��ͼ��ʾ��
��֪A��B��C��D��Ϊ���壬E��F��Ϊ�����³ʹ�������ӻ����GΪ�Ȼ��ƣ�A��B��ȼ�յĻ���ʲ�ɫ����Ӧ���������������д������̣�����֮���ת����ϵ��ͼ��ʾ��


