��Ŀ����
ij����С��ͬѧ����ͼװ�ý���ʵ�飬һ��ʱ�����C�缫������ͭ�������Իش��������⡣
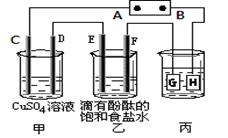
��1��AΪ��Դ�� ����
��2��E�ĵ缫��ӦʽΪ�� ��
��3���ڳ����£����ñ�װ�ø�����ͭ������������������ͭ��3.2gʱ��������Һ��PHֵΪ ��������Һ���Ϊ1L����
��4���ڵ��һ��ʱ����ڼ��м������� ����ʹ��Һ�ָ���ԭ����Ũ�ȡ�
��5�����÷�Ӧ2Cu��O2��2H2SO4=2CuSO4��2H2O���Ʊ�CuSO4�������÷�Ӧ���Ϊԭ��أ��������缫��ӦʽΪ______________��

��1��AΪ��Դ�� ����
��2��E�ĵ缫��ӦʽΪ�� ��
��3���ڳ����£����ñ�װ�ø�����ͭ������������������ͭ��3.2gʱ��������Һ��PHֵΪ ��������Һ���Ϊ1L����
��4���ڵ��һ��ʱ����ڼ��м������� ����ʹ��Һ�ָ���ԭ����Ũ�ȡ�
��5�����÷�Ӧ2Cu��O2��2H2SO4=2CuSO4��2H2O���Ʊ�CuSO4�������÷�Ӧ���Ϊԭ��أ��������缫��ӦʽΪ______________��
��8�֣���1��������1�֣� ��2��2H����2e����H2����2�֣� ��3��13 ��2�֣�
��4��CuO��CuCO3[�����ǵ�ˮ��ʼ���Cu��OH��2Ҳ����]��1�֣���5��4H����O2��4e����2H2O��2�֣�
��4��CuO��CuCO3[�����ǵ�ˮ��ʼ���Cu��OH��2Ҳ����]��1�֣���5��4H����O2��4e����2H2O��2�֣�
�����������1��һ��ʱ�����C�缫������ͭ��������˵��C�缫����������Һ�е�ͭ���ӷŵ������ͭ������A�缫�ǵ�Դ�ĸ�����
��2��E�缫�͵�Դ�ĸ���������������������Һ�е������ӷŵ磬�缫��ӦʽΪ2H����2e����H2����
��3��3.2gͭ�����ʵ�����3.2g��64g/mol��0.05mol��ת��0.05mol��2��0.1mol���ӡ����ݵ�ʧ�����غ��֪���ҳ���Ҳת��0.1mol���ӡ����Ը��ݷ���ʽ2NaCl��2H2O
 2NaOH��H2����Cl2����֪��ÿ����1mol�������Ʒ�Ӧ�о�ת��1mol���ӣ���˷�Ӧ�����ɵ�����������0.1mol����Ũ����0.1mol/L��������Һ��������Ũ����10��13mol/L����pH��13��
2NaOH��H2����Cl2����֪��ÿ����1mol�������Ʒ�Ӧ�о�ת��1mol���ӣ���˷�Ӧ�����ɵ�����������0.1mol����Ũ����0.1mol/L��������Һ��������Ũ����10��13mol/L����pH��13����4�����Ե缫��⣻����ͭ��Һ����������������ϡ�����ͭ�������ٵ�����ԭ�Ӻ�ͭԭ�ӣ�����Ҫʹ��Һ�ָ���ԭ����Ũ��Ӧ������м�������CuO��CuCO3��
��5��ԭ����и���ʧȥ���ӣ������õ����ӡ����ݷ�Ӧʽ2Cu��O2��2H2SO4=2CuSO4��2H2O��֪�������õ����ӣ���������������õ����ӡ�������Һ�����ԣ��������缫��ӦʽΪ4H����O2��4e����2H2O��

��ϰ��ϵ�д�
�����Ŀ


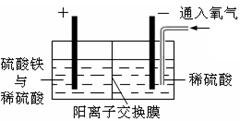
 R2Cu(�л���)+2H��(ˮ��)������л��࣬�����м���һ��Ũ�ȵ����ᣬʹCu2+���������� �����������ͭ��Һ�Ƶý���ͭ��
R2Cu(�л���)+2H��(ˮ��)������л��࣬�����м���һ��Ũ�ȵ����ᣬʹCu2+���������� �����������ͭ��Һ�Ƶý���ͭ��