题目内容
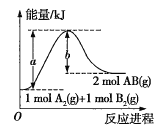
【题目】现有14.4 g CO和CO2的混合气体,在标准状况下所占的体积约为8.96 L。回答下列问题:
(1)该混合气体的平均摩尔质量:_________________________。
(2)混合气体中碳原子的个数:_________________________
(用NA表示阿伏加德罗常数的值)。
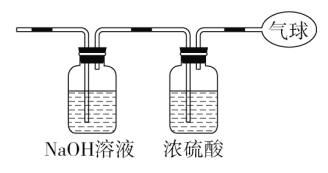
(3)将混合气体依次通过如图装置,最后收集在气球中。
①气球中收集到的气体摩尔质量:_______________________;
②标况下气球中收集到的气体的体积为___________________;
③气球中收集到的气体的电子总数为_____________________
(用NA表示阿伏加德罗常数的值)。
【答案】36 g·mol-1 0.4NA 28 g·mol-1 4.48 L 2.8NA
【解析】
(1)混合气体的体积为8.96 L,则其物质的量为n=![]() =
=![]() =0.4 mol,混合气体的平均摩尔质量为
=0.4 mol,混合气体的平均摩尔质量为![]() =36 g·mol-1;
=36 g·mol-1;
(2)设混合气体中 CO的物质的量为x mol,CO2的物质的量为y mol,则根据混合物的质量为14.4 g可得:28x+44y=14.4;①;根据气体的物质的量为0.4 mol,所得x+y=0.4②;解①②得:x=0.2 mol,y=0.2 mol;由于CO和CO2中均含1个碳原子,故0.2 mol CO和0.2 mol CO2中共含0.4 mol C原子即0.4NA个;
(3)将混合气体依次通过如图装置,则CO2会被NaOH溶液吸收,剩余CO,被浓硫酸干燥后,则在气球中收集到的是干燥纯净的CO气体;
①气球中收集到的气体为CO,而一种物质的摩尔质量在数值上等于该物质的相对分子质量,故收集到的气体的摩尔质量为28 g·mol-1;
②气球中的气体为CO,其体积V=nVm=0.2 mol×22.4 L·mol-1=4.48 L;
③1个CO含有14个电子,由(2)求出的CO的物质的量为0.2 mol,则电子的物质的量为0.2 mol×14=2.8 mol,电子总数为2.8NA个。

【题目】探究铝片与Na2CO3溶液的反应:
| | |
无明显现象 | 铝片表面产生细小气泡 | 出现白色浑浊,产生大量气泡(经检验为 H2和CO2) |
下列说法正确的是( )
A.Na2CO3溶液中存在水解平衡:CO32-+2H2O![]() H2CO3+2OH-
H2CO3+2OH-
B.推测出现白色浑浊的原因:AlO2-+HCO3-+H2O=Al(OH)3↓+CO2↑
C.对比Ⅰ、Ⅲ,说明 Na2CO3溶液能破坏铝表面的保护膜
D.加热和H2逸出对CO32-水解平衡移动方向的影响是相反的
【题目】铋酸钠(NaBiO3)是分析化学中的重要试剂,在水中缓慢分解,遇沸水或酸则迅速分解。某兴趣小组设计实验制取铋酸钠并探究其应用。回答下列问题:
Ⅰ.制取铋酸钠
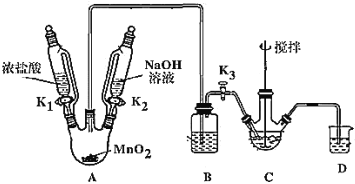
制取装置如图(加热和夹持仪器已略去),部分物质性质如下:
物质 | NaBiO3 | Bi(OH)3 |
性质 | 不溶于冷水,浅黄色 | 难溶于水;白色 |
(1)装MnO2的仪器名称是_____________,B装置用于除去HCl,盛放的试剂是________________;
(2)C中盛放Bi(OH)3与NaOH混合物,与Cl2反应生成NaBiO3,反应的离子方程式为_____;
(3)当观察到_____(填现象)时,可以初步判断 C 中反应已经完成;
(4)拆除装置前必须先除去烧瓶中残留Cl2以免污染空气,除去Cl2的操作是_______。
(5)反应结束后,为从装置C中获得尽可能多的产品,需要的操作是____________,过滤、洗涤、干燥;
Ⅱ.产品纯度的测定
(6)取上述NaBiO3产品w g,加入足量稀硫酸和MnSO4稀溶液使其完全反应,再用 c mo1/L的H2C2O4标准溶液滴定生成的MnO4-(已知:H2C2O4+MnO4---CO2+Mn2++H2O,未配平),当溶液紫红色恰好褪去时,消耗V mL标准溶液。该产品的纯度为 ______________(用含 w、c、V的代数式表示)。