��Ŀ����
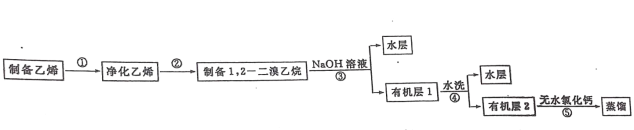
����Ŀ��ijѧϰС��������ϣ��������������֤ SO2 �Ļ�ԭ�ԡ�
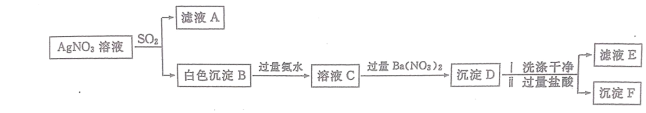
��֪������ D ϴ�Ӹɾ��������������ʱ�����ֳ����ܽ⣬ʣ���������� F��(1)��������ɫ���� B���ijɷ�
����ͬѧ�ƶϰ�ɫ���� B �� Ag2SO4��
ʵ���������ݣ������� F��˵�������� D���к�________________ (�ѧʽ)������˵������ɫ����B���к� Ag2SO4��
����ͬѧ��Ϊ����ɫ���� B���к��� Ag2SO3��
ʵ���������ݣ������� D ϴ�Ӹɾ��������������ʱ�����ֳ����ܽ������ܽ������Ϊ BaSO3�� �����ƶϳ��� B �к� Ag2SO3��Ϊ��һ��֤ʵ B �к��� Ag2SO3����ȡ������Һ E���Թ��м�������________________���а�ɫ�������ɡ�
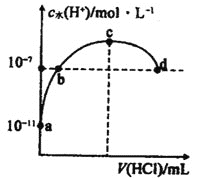
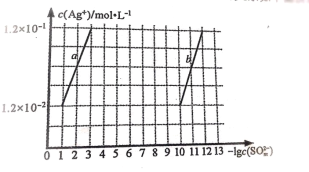
(2)�����£�Ag2SO4 �� Ag2SO3 �� c(Ag+)�� c(SO32-)�Ĺ�ϵ����ͼ��ʾ��
��֪��Ksp(Ag2SO4)��Ksp(Ag2SO3)���ش����⣺
����ʾ Ag2SO4 �ij����ܽ�ƽ���ϵ��������________________ (����a������b��)
��Ksp(Ag2SO3)Ϊ________________mol3L-3
���𰸡�BaSO4 H2O2������ˮ����ˮ���������� a 1.44��10-14
��������
�Ţ��������֪����ҺC�������ᱵ���ɵij���D���������ϡ���ᣬ��δ�ܽ�ij���F�������FΪBaSO4�������Dһ����BaSO4��
����������֪������D��δ�ܽ��ΪBaSO4���ܽ��ΪBaSO3��BaSO3��HCl��Ӧ����BaCl2��SO2��ֻҪ֤����ҺE�к���SO32������֤��B�к���Ag2SO3������ѡ�õ��Լ���H2O2��ǿ�����Ե���Һ��
(2)�ٵ�������Ũ����ͬʱ�����������Ũ�ȴ��������������Ũ�ȣ���˱�ʾAg2SO4�ij����ܽ�ƽ���ϵ��������a��
��Ksp(Ag2SO3)Ϊ![]() ��
��
�Ţ��������֪����ҺC�������ᱵ���ɵij���D���������ϡ���ᣬ��δ�ܽ�ij���F�������FΪBaSO4�������Dһ����BaSO4���ʴ�Ϊ��BaSO4��
����������֪������D��δ�ܽ��ΪBaSO4���ܽ��ΪBaSO3��BaSO3��HCl��Ӧ����BaCl2��SO2��ֻҪ֤����ҺE�к���SO32������֤��B�к���Ag2SO3������ѡ�õ��Լ���H2O2��ǿ�����Ե���Һ���ʴ�Ϊ������H2O2������ˮ����ˮ������������
(2)�ٵ�������Ũ����ͬʱ�����������Ũ�ȴ��������������Ũ�ȣ���˱�ʾAg2SO4�ij����ܽ�ƽ���ϵ��������a���ʴ�Ϊ��a��
��Ksp(Ag2SO3)Ϊ![]() ���ʴ�Ϊ��1.44��10-14��
���ʴ�Ϊ��1.44��10-14��