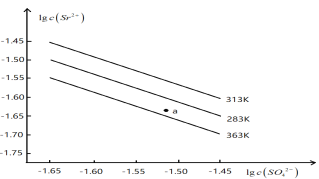
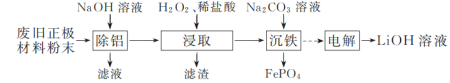
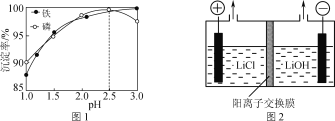
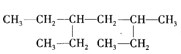��Ŀ����
����Ŀ������������Ԫ��X��Y��Z��W��ԭ��������������X��Wͬ���壬Yԭ�ӵ����������������ڲ��������3����0.1 mol Z�������������ᷴӦ����H2�����Ϊ3.36 L������ɱ�״������W��Ԫ�����ڱ��е���������������������1������˵����ȷ���ǣ�������
A.XY2�������ӻ�����
B.X��һ�ֵ����ڸ�������WY2��Ӧ���Ƶ�W����
C.ԭ�Ӱ뾶��r��Z����r��W����r��Y����r��X��
D.W�ļ���̬�⻯������ȶ��Ա�Y��ǿ
���𰸡�B
��������
����������Ԫ��X��Y��Z��W��ԭ��������������X��Wͬ���壬Yԭ�ӵ����������������ڲ��������3����YΪOԪ�أ�0.1 mol Z�������������ᷴӦ����H2�����Ϊ3.36 L������ɱ�״������H2�����ʵ���Ϊ![]() mol=0.15mol,0.1Ħ�����������ᷴӦ����0.15Ħ����H2,��1Ħ�����������ᷴӦ����1.5Ħ����H2,���Խ����Ļ��ϼ�Ϊ+3��,ZΪAlԪ�أ�W��Ԫ�����ڱ��е���������������������1��WΪSiԪ�أ�XΪCԪ�ء�
mol=0.15mol,0.1Ħ�����������ᷴӦ����0.15Ħ����H2,��1Ħ�����������ᷴӦ����1.5Ħ����H2,���Խ����Ļ��ϼ�Ϊ+3��,ZΪAlԪ�أ�W��Ԫ�����ڱ��е���������������������1��WΪSiԪ�أ�XΪCԪ�ء�
A��CO2���ڹ��ۻ������A����
B��C�ڸ�������SiO2��Ӧ���Ƶ�Si����ͬʱ����CO����B��ȷ��
C��ͬһ����,������ԭ������������,ԭ�Ӱ뾶Խ��ԽС��ͬ������ϵ���ԭ�Ӱ뾶Խ��Խ��ԭ�Ӱ뾶��r��Z����r��W����r��X����r��Y����r��Al����r��Si����r��C����r��O������C����
D��ͬ���ڴ�������Ԫ�صķǽ�������ǿ��ͬ����Ԫ�ش��ϵ���Ԫ�صķǽ����Լ������ɷǽ�����Խǿ�����Ӧ���⻯����ȶ���Խ�ǽ�����O>S>Si��SiH4���ȶ���С��H2O����D����
��ѡB��

 ���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
���ɿ��õ�Ԫ������ĩר����100��ϵ�д�����Ŀ��ͭ��(��Ҫ�ɷ� CuSO4��5H2O)��һ�ֿ�����ʳƷ���ӵ�ͭǿ����������ij��������(���� CuSO4��CuSO3��Cu2O���������������Cu2S��CuS)�Ʊ�ͭ���Ĺ��չ������£�
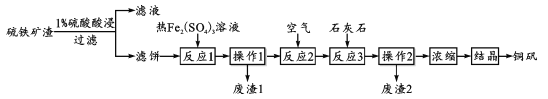
(1)��1%���������ʱ����Һ������Ϊ1:3������4��6�ν�ȡ����Ŀ����_________��
(2)���˱����к���Cu������Cu�ڡ���Ӧ1�����ܽ�����ӷ���ʽΪ________��������1����ֻ����S���ʣ���Ӧ1����Cu2S��Fe2(SO4)3��Ӧ�����ʵ���֮��Ϊ_______��
(3)����Ӧ2����ͨ�������Ŀ����_______��������ӷ���ʽ��˵������Ӧ3������ʯ��ʯ������________��
(4)Ϊ��������������������ʺͲ�Ʒ�IJ��ʣ��ڡ�Ũ����ǰ���еı�Ҫ������_____�����������ܽ����Ϣ�������˵Ľᾧ��ʽΪ_________��
t/�� | 0 | 10 | 20 | 30 | 40 | 60 | 80 |
CuSO4��5H2O/(g/100g H2O) | 23.1 | 27.5 | 32.0 | 37.8 | 44.6 | 61.8 | 83.8 |
(5)��ͭ������ʯ�ҡ�ˮ������������Ϊ1.0:0.56:100���������ͭɱ����������Һ������Ч�ɷ�ΪCuSO4��xCu(OH)2��yCa(OH)2����x=1ʱ����ȷ��y��ֵΪ____��
����Ŀ����25��ʱ���ܱ�������X��Y��Z��������ij�ʼŨ�Ⱥ�ƽ��Ũ�����±���
���� | X | Y | Z |
��ʼŨ��/mol��L-1 | 0.1 | 0.2 | 0 |
ƽ��Ũ��/mol��L-1 | 0.05 | 0.05 | 0.1 |
����˵���������
A.��Ӧ�ﵽƽ��ʱ��X��ת����Ϊ50��
B.����ѹǿʹƽ��������Z�ķ����ƶ�
C.��25���£���Ӧ��ƽ�ⳣ��Ϊ1600���ı��¶ȿ��Ըı�˷�Ӧ��ƽ�ⳣ��
D.��25���£����c(X)=0.04mol��L-1��c(Y)=0.1mol��L-1��c(Z)=0.08mol��L-1�����ʱv��<v��