��Ŀ����
����Ŀ������Ļ��������Ź㷺����;����ش��������⣺

(1)����Ԫ�����ڱ��е�λ��Ϊ��____________________________________��
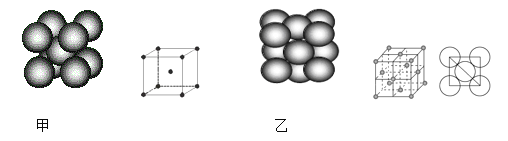
(2)��þ��39Kʱ�ʳ����ԣ�����þ���������ģ���У�þԭ�Ӻ���ԭ���Ƿֲ��Ų��ģ�һ��þһ����������С�ͼa�Ǹþ����ۿռ���ȡ���IJ���ԭ����Z�᷽���ͶӰ��������þԭ��ͶӰ����������ԭ��ͶӰ��ͶӰ��ͬһƽ���ϡ�����ͼʾȷ����þ�Ļ�ѧʽΪ_____________��
(3)����(H3BO3)����ɫƬ״���壬����ˮ���������������֯�з����������á�H3BO3��Һ�д���H3BO3(aq)��H2O(l)![]() [B(OH)4]��(aq)��H��(aq)��ƽ�⡣���������NaOH��Һ��Ӧ����ʽΪ��H3BO3��NaOH��Na[B(OH)4]�����ᾧ����в�״�ṹ��ÿһ��ṹ��ͼ1��ʾ��
[B(OH)4]��(aq)��H��(aq)��ƽ�⡣���������NaOH��Һ��Ӧ����ʽΪ��H3BO3��NaOH��Na[B(OH)4]�����ᾧ����в�״�ṹ��ÿһ��ṹ��ͼ1��ʾ��
�����ᾧ������_______����(������������������������ԭ����)��
�����й��������˵����ȷ����_______________(�����)��
a.H3BO3��һԪ��
b.��ˮ��Һ�У�ˮ�ĵ���ƽ���ܵ�����
c.����������������������ӽ��ȶ�
(4)��������(BF3)ˮ����������ͷ�����(HBF4��ǿ��)��BF3�������幹��Ϊ___________��BF4���ĵ���ʽ____________��
(5)������(NBH6)��һ����Ч����ȫ�Ĺ��崢����ϡ�������Ľṹ(��ͼb)���������ƣ�����������������������ԭ�ӵ��ӻ�������ͷֱ�Ϊ��___________��___________��
���𰸡��ڶ����ڢ�A�� MgB2 ���� a ƽ��������  sp3 sp2
sp3 sp2
��������
����5��Ԫ�أ�λ�ڵڶ����ڵ���A�壻��ͼa֪1��Bԭ��Ϊ3��Mgԭ�ӹ��ã�1��Mgԭ��Ϊ6��Bԭ�ӹ��ã����Լ�����ԭ�Ӻ�þԭ�ӵø���֮�ȣ����ᾧ���д���H3BO3���ӣ��Ƿ��Ӿ��壻������Bԭ������ȱ����ԭ�ӣ���ˮ��Һ�п��Խ��ˮ���������������H3BO3(aq)��H2O(l)![]() [B(OH)4]��(aq)��H��(aq)�����ԣ�����ļ��룬�ٽ���ˮ�ĵ��룬������������������ʷ��棻��������3�����ӣ�����д����ԭ�ӵ�����������4��F��ϣ���һ��F����B�γɹ��ۼ�ʱ��Ҫ��һ�����ӣ�����������-1�ۣ����ݼ۲���ӶԻ������ۣ����Լ�������ԭ�ӵŵ��Ӷ������Ӷ��ó��ӻ���ʽ��������Ľṹ(��ͼb)���������ƣ�����ԭ�ӵ��ӻ���ʽ�������е�̼һ����
[B(OH)4]��(aq)��H��(aq)�����ԣ�����ļ��룬�ٽ���ˮ�ĵ��룬������������������ʷ��棻��������3�����ӣ�����д����ԭ�ӵ�����������4��F��ϣ���һ��F����B�γɹ��ۼ�ʱ��Ҫ��һ�����ӣ�����������-1�ۣ����ݼ۲���ӶԻ������ۣ����Լ�������ԭ�ӵŵ��Ӷ������Ӷ��ó��ӻ���ʽ��������Ľṹ(��ͼb)���������ƣ�����ԭ�ӵ��ӻ���ʽ�������е�̼һ����
(1) ����5��Ԫ�أ�λ�ڵڶ����ڵڢ�A�壻
�ʴ�Ϊ���ڶ����ڵڢ�A�壻
(2) ��ͼa֪1��Bԭ��Ϊ3��Mgԭ�ӹ��ã�����һ��Mgԭ�ӵ�Bԭ��Ϊ![]() ��1��Mgԭ��Ϊ6��Bԭ�ӹ��ã�����һ��Bԭ�ӵ�Mgԭ��Ϊ
��1��Mgԭ��Ϊ6��Bԭ�ӹ��ã�����һ��Bԭ�ӵ�Mgԭ��Ϊ![]() �����Լ�����ԭ�Ӻ�þԭ�ӵø���֮�ȣ�2��1������þ�Ļ�ѧʽΪ��MgB2��
�����Լ�����ԭ�Ӻ�þԭ�ӵø���֮�ȣ�2��1������þ�Ļ�ѧʽΪ��MgB2��
�ʴ�Ϊ��MgB2��
(3) �����ᾧ���д���H3BO3���ӣ��Ƿ��Ӿ��壻��������Bԭ������ȱ����ԭ�ӣ���ˮ��Һ�п��Խ��ˮ���������������H3BO3(aq)��H2O(l)![]() [B(OH)4]��(aq)��H��(aq)����һԪ�����ļ��룬�ٽ���ˮ�ĵ��룻������������������ʷ��棬���۷е�ȣ�
[B(OH)4]��(aq)��H��(aq)����һԪ�����ļ��룬�ٽ���ˮ�ĵ��룻������������������ʷ��棬���۷е�ȣ�
�ʴ�Ϊ�����ӣ�a��
(4) ���ݼ۲���ӶԻ������ۣ����Լ�������ԭ�ӵŵ��Ӷ�����![]() ���µ��Ӷ������ʷ��ӵ����幹��Ϊ��ƽ�������Σ���������3�����ӣ�����д����ԭ�ӵ�����������4��F��ϣ���һ��F����B�γɹ��ۼ�ʱ��Ҫ��һ�����ӣ�����������-1�ۣ�����ʽΪ��
���µ��Ӷ������ʷ��ӵ����幹��Ϊ��ƽ�������Σ���������3�����ӣ�����д����ԭ�ӵ�����������4��F��ϣ���һ��F����B�γɹ��ۼ�ʱ��Ҫ��һ�����ӣ�����������-1�ۣ�����ʽΪ��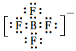 ��
��
�ʴ�Ϊ��ƽ�������Σ� ��
��
(5)������Ľṹ(��ͼb)���������ƣ�����ԭ�ӵ��ӻ���ʽ�������е�̼һ����Ϊsp3�ӻ��������п���ͨ����ʽ�������������![]() ��������������ԭ��Ϊsp2�ӻ���
��������������ԭ��Ϊsp2�ӻ���
�ʴ�Ϊ��sp3 �� sp2��