��Ŀ����
��һ��ϸ��������ˮ��Һ�����������£����Խ���ͭ����Ҫ�ɷ���CuFeS2������������SiO2�������������Ρ����ø�ԭ������ͭ���̷���FeSO4��7H2O�����������£�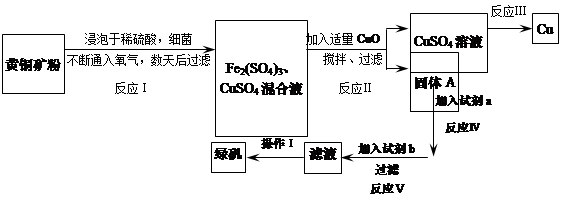
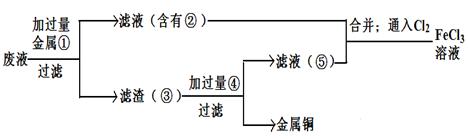
�ش��������⣺
��1����֪��
| | Fe2+ | Cu2+ | Fe3+ |
| ��ʼת���������������ʱ��pH | 7.6 | 4.7 | 2.7 |
| ��ȫת���������������ʱ��pH | 9.6 | 6.7 | 3.7 |
��1������CuO����Һ��pH���ڵ�ԼΪ4�����ƽ���ƶ�ԭ�����ò�����ԭ�� ��
��2��д����ʵ�ַ�Ӧ��Ļ�ѧ����ʽ ��
��3���Լ�bΪ ��
��4��������Һ�л���̷����壬������ӦΪŨ���� �� ��
��5����Ӧ��Ļ�ѧ����ʽ ��
��1��Fe3+����Һ�д���ˮ��ƽ�⣬��Fe3++ 3H2O Fe��OH��3+3H+����PHС��4ʱ��Fe3+��ʼ������PH=2.7��������������ͭ������ͭ����H+��Ӧ���ٽ�ˮ��ƽ�����ƣ�ʹFe3+ת��ΪFe��OH��3����ȥ��������ͭ������ģ��Ҳ������������ʡ�
Fe��OH��3+3H+����PHС��4ʱ��Fe3+��ʼ������PH=2.7��������������ͭ������ͭ����H+��Ӧ���ٽ�ˮ��ƽ�����ƣ�ʹFe3+ת��ΪFe��OH��3����ȥ��������ͭ������ģ��Ҳ������������ʡ�
��2��2CuSO4+2 H2O 2Cu+O2+2H2SO4.��Fe+CuSO4=Cu+FeSO4
2Cu+O2+2H2SO4.��Fe+CuSO4=Cu+FeSO4
(3) Fe����4���ᾧ�����ˣ�5��4CuFeS2+17O2+2H2SO4�T2Fe2��SO4��3+4CuSO4+2H2O
���������������1��Fe3+����Һ�д���ˮ��ƽ�⣬��Fe3++ 3H2O Fe��OH��3+3H+����PHС��4ʱ��Fe3+��ʼ������PH=2.7��������������ͭ������ͭ����H+��Ӧ���ٽ�ˮ��ƽ�����ƣ�ʹFe3+ת��ΪFe��OH��3����ȥ��������ͭ������ģ��Ҳ������������ʡ�
Fe��OH��3+3H+����PHС��4ʱ��Fe3+��ʼ������PH=2.7��������������ͭ������ͭ����H+��Ӧ���ٽ�ˮ��ƽ�����ƣ�ʹFe3+ת��ΪFe��OH��3����ȥ��������ͭ������ģ��Ҳ������������ʡ�
��2���������ͭ��Һ����ѧ����ʽΪ��2CuSO4+2 H2O 2Cu+O2��+2H2SO4.��Fe+CuSO4=Cu+FeSO4
2Cu+O2��+2H2SO4.��Fe+CuSO4=Cu+FeSO4
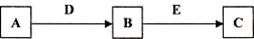
��3����һ��Ӧ�ӹ�������ͼ�Ľṹ���֣�ͨ����1���ķ������Ѿ�֪������AΪ���������������Լ�a��õ�����Һͨ����Ӧv����b����Һ�е�������ת��ΪFeSO4���������ʵ�ת��ԭ����֪bΪFe��
��4������̷�����ʱ������Ũ������Ϊ���������ܽ�����¶ȵ����߶��������Խ����ܽ�ȼ�С�����������壬�����˼��ɣ�
��5����Ӧ���еķ�Ӧ����CuFeS2��O2��H2SO4�������ʣ���������������������ͭ��ˮ��������ż����ƽ�����Է���ʽ�ǣ�4CuFeS2+17O2+2H2SO4�T2Fe2��SO4��3+4CuSO4+2H2O��
���㣺�������ʵķ�����ᴿ�����ʵ��ת�����Ʊ����ᾧԭ������������Ӧ�á������Ļ�ѧ���ʡ���д��ѧ����ʽ�����������ȡ�

 ����ʦ���һ��һ��ϵ�д�
����ʦ���һ��һ��ϵ�д� �Ͻ�ƽ��У����ϵ�д�
�Ͻ�ƽ��У����ϵ�д���1��Ԫ��M�Ƕ�����Ԫ�أ��䳣�������ں�ˮ�У����ʱ���Ϊ��������������
��M��ԭ�ӽṹʾ��ͼΪ______��
����M��AlΪ�缫��KOH��ҺΪ�������Һ�����ĵ缫��ӦʽΪ______��
��2������ǽ������������ȵ�ij�¶ȣ��漴�����������п�����ȴ�Ľ����ȴ������ա�
��ʹ��ˮ���д�������ɴ������������÷�Ӧ�Ļ�ѧ����ʽΪ____________
����֤����ˮ����Ĺ�������Ƿ����+3�۵�������ѡ�õ��Լ�Ϊ_______ (�����)
| A��H2O2��Һ | B��ͭ�� | C��ϡ���� | D��KMnO4��Һ |
 4Fe(OH)3+8OH��+3O2
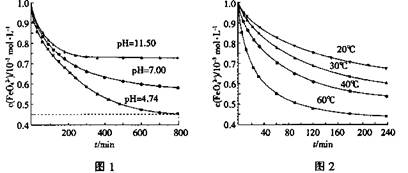
4Fe(OH)3+8OH��+3O2��ͼ1��25��ʱK2FeO4�ڲ�ͬpH��Һ��Ũ�ȵı仯�����pH =4.74ʱ����Ӧ�ӿ�ʼ��800min��ƽ����Ӧ����v(FeO42��)=______ (������λ��Ч���֣���
��ͼ1��800min��������Һ��K2FeO4��Ũ�Ⱦ����ٸı䡣�۲�ͼ1�ж�����pH ��˷�Ӧ��ƽ�ⳣ��______����������С�����䡱����
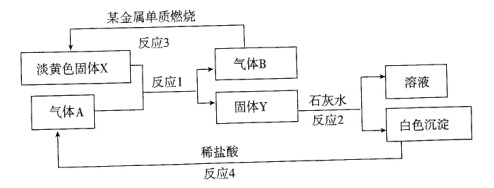
��ͼ2��240min��������Һ��K2FeO4��Ũ�Ⱦ����ٸı䣬��������Ӧ�ķ�Ӧ�ȡ�H______0(�>������<������=������