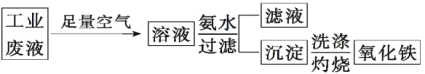
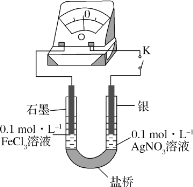
��Ŀ����
����Ŀ���±���ij����ij�տ����������棺
��Ⱦָ�� | ��Ҫ��Ⱦ�� | ������������ | ��������״�� |
55 | SO2 | II | �� |
����ijУ�о���ѧϰС��Ա�����Ҫ��Ⱦ��SO2��������ij������̽����
ʵ��һ������ͼ��ʾװ�ý���ʵ�顣
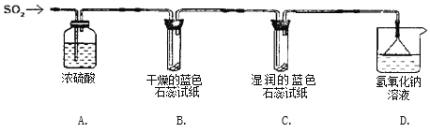
(1)Aװ�õ�������_________������������������������SO2���塣
(2)ʵ������У�Bװ����ʯ����ֽ����ɫû�з����仯��Cװ����ʪ�����ɫʯ����ֽ��_______ɫ(������ɫ��������ɫ����������ɫ��)��˵��SO2��ˮ��Ӧ����һ���ᡣ
(3)Dװ�õ�������______________����д����ѧ��Ӧ����ʽ_____________________________��
ʵ�������ʢ��ˮ���ձ���ͨ��SO2���壬���������Һ��pH_______7����������������������������Ȼ��ÿ��1 h�ⶨ��pH������pH��С��ֱ���㶨��˵���ձ�����Һ�������е�����������������H2SO4��
���������ϣ� SO2�γ��������һ;����SO2������е�O2��Ʈ���������·�Ӧ����SO3��SO3���ڽ�ˮ����H2SO4���ڴ˹�����Ʈ����_________����������������������������
��̽�����ۣ�SO2������е�������ˮ��Ӧ���������γ����ꡣ���п����׳������֪꣨ʶ���룩
(1)������ɵ�Σ���ǣ�______________________________����һ������
(2)�����ŷŵ�β�������ᡢ���ʵȹ�ҵ�����ų��ķ����ж����е����������������������ˮ����ת��Ϊ____________��������������һ��Ҫԭ��
���𰸡����� ��ɫ ���� SO2���� SO2 + 2NaOH=Na2SO3 + H2O < ���� ��ʴ�����������Ʒ�� HNO3�����ᣩ
��������
ʵ��һ������Ũ���Ὣ����������Ȼ��Bװ�ü������Ķ��������Ƿ������ԣ�Cװ�ü���ʪ��Ķ��������Ƿ������ԣ�Dװ�ý���β������������©����������
ʵ��һ��
(1)����������Ũ�����Ӧ��Ũ��������ˮ�ԣ��������ն��������е�ˮ����������Ũ���������Ϊ����������������壬
(2)װ�� B ����ɫʯ����ֽ��Ȼ�������������Ӵ�����ȴ����ɫ������˵�������������岻��ʹ�������ɫ��ֽ��ɫ����C װ����ʪ�����ɫʯ����ֽ��죬˵����ֽ�������������ʣ�������������ʹ��ֽ��죬��ˣ����ƶ϶���������ˮ�����γ������ʹ��ֽ��죻
(3)����������һ����ɫ���д̼�����ζ���ж����壬β����Ҫ����������������������Һ��Ӧ��������������ˮ����Ӧ�Ļ�ѧ����ʽΪ��SO2 + 2NaOH=Na2SO3 + H2O��
ʵ��������������ˮ��Ӧ���������ᣬ��Һ�����ԣ�pH��7��
SO2������е� O2��Ʈ���������·�Ӧ���� SO3��SO3���ڽ�ˮ���� H2SO4���ڴ˹�����Ʈ����������
(1)��������ԣ����������ܹ���ʴ��Ҫ�ɷ���̼���λ�����Ľ��������ȣ�ͬʱҲ�ܻٻ� ɭ����ľ��ʹˮ�塢�����ữ��
(2)�����������ڿ����з�����Ӧ��2NO+O2=2NO2��3NO2+H2O=2HNO3+NO��4NO2+O2+2H2O�T4HNO3�� 4NO+3O2+2H2O�T4HNO3�����Ե�������������ˮ����ת��Ϊ���

 �����ߴ���ϵ�д�
�����ߴ���ϵ�д�