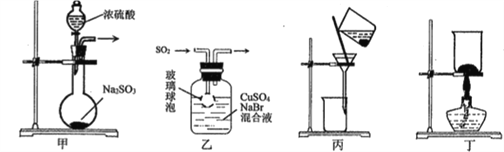
��Ŀ����
����Ŀ����8�֣���Ba(OH)2��Һ����μ���ϡ���ᣮ������������⣺
��1��д����Ӧ�����ӷ���ʽ_____________��
��2��������������£����ӷ���ʽ�루1����ͬ����________������ţ���
A����NaHSO4��Һ�У���μ���Ba(OH)2��Һ����Һ������
B����NaHSO4��Һ�У���μ���Ba(OH)2��Һ��SO42��ǡ����ȫ����
C����NaHSO4��Һ�У���μ���Ba(OH)2��Һ������
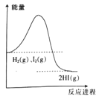
��3������������ϡH2SO4ֱ�����������������л����Һ�еĵ����������õ���ǿ��I��ʾ���ɽ��Ƶ���ͼ�е����߱�ʾ��________������ţ���

��4������װ��Ba(OH)2��Һ�ձ��ﻺ������KAl(SO4)2��Һ��Ba2+ǡ����ȫ��Ӧ����Ӧ�����ӷ���ʽ�� _________________��
���𰸡���8�֣�ÿ��2�֣���1��Ba2++2OH-+2H++SO42-=BaSO4��+2H2O�� ��2��A �� ��3��C ��
��4��2Ba2++4OH-+Al3++2SO42-=2BaSO4 �� +AlO2-+2H2O��
��������
������Ba(OH)2��Һ����μ���ϡ���ᣬ�����ᡢ���кͷ�Ӧ�������κ�ˮ����Ӧ�����ӷ���ʽ��Ba2++2OH-+2H++SO42-=BaSO4��+2H2O����2��A����NaHSO4��Һ�У���μ���Ba(OH)2��Һ����Һ�����ԣ���Ӧ�����ӷ���ʽ��Ba2++2OH-+2H++SO42-=BaSO4��+2H2O����ȷ��B����NaHSO4��Һ�У���μ���Ba(OH)2��Һ��SO42��ǡ����ȫ��������Ӧ�����ӷ���ʽ�ǣ�Ba2++OH-+H++SO42-=BaSO4��+H2O������C����NaHSO4��Һ�У���μ���Ba(OH)2��Һ��������Ҫ�Բ�������NaHSO4��ҺΪ������Ӧ�����ӷ���ʽ�ǣ�Ba2++OH-+H++SO42-=BaSO4��+H2O������������(1)���ӷ���ʽ��ͬ����A����3����������Ba(OH)2��Һ�м���ϡH2SO4ֱ�����������ڷ�����Ӧ��Ba2++2OH-+2H++SO42-=BaSO4��+2H2O��ʹ��Һ�������ƶ������ӵ����ʵ���Ũ�ȼ�С����Һ�ĵ�����������������ǡ����ȫ��Ӧʱ����Һ�������ƶ�������Ũ����С������H2O�Ǽ����ĵ���ʣ��������������Ũ�Ⱥ�С��BaSO4���ܣ��ܽ�������������Ũ��Ҳ��С����ʱ��Һ������Ũ�ȼ���Ϊ0�����������ʱ������������������ʹ��Һ�������ƶ�������Ũ��������Һ�ĵ�����������ǿ��������������л����Һ�еĵ����������õ���ǿ��I��ʾ���ɽ��Ƶ�����ͼ�е����߱�ʾ��C����4������װ��Ba(OH)2��Һ�ձ��ﻺ������KAl(SO4)2��Һ��Ba2+ǡ����ȫ��Ӧ����ʱ�������ʵ����ʵ����ı���n[Ba(OH)2]:n[ KAl(SO4)2] =2:1������Al(OH)3��������������ɱ�������ǿ���ܽ⣬��Ӧ�����ӷ���ʽ��2Ba2++4OH-+Al3++2SO42-=2BaSO4 �� +AlO2-+2H2O��

����Ŀ����ij��Br����ˮ����ȡBr2�Ĺ��̰��������ˡ���������ȡ����ѡ�������ȡ����������Ȳ��衣��֪��
���� | Br2 | CCl4 | ��ʮ���� |
�ܶ�/g��cm-3 | 3.119 | 1.595 | 0.753 |
�е�/�� | 58.76 | 76.8 | 215~217 |
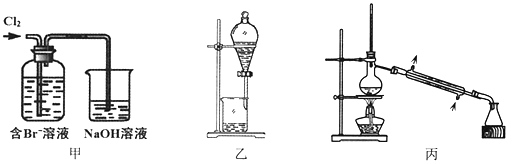
����˵������ȷ����
A. ��װ����Br�������ķ�ӦΪ��2Br-+ Cl2 = Br2 + 2Cl-
B. ��װ����NaOH��Һÿ����0.1mol Cl2��ת��0.1mol e��
C. ����װ�ý�����ȡ���ܽ�Br2���л������²�
D. �ñ�װ�ý����������ռ�������Br2