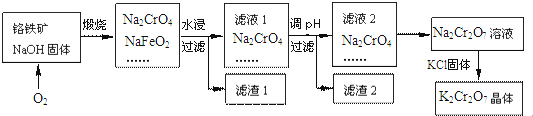
��Ŀ����
3��ʵ������10mol•L-1��Ũ��������450mL 1mol•L-1��ϡ���ᣬ�ش��������⣺��1����ȡŨ��������Ϊ50.0mL��Ӧѡ�õ���Ͳ���Ϊ50mL��
��2������ʱ��Ҫ�õ����������Լ�ƿ����Ͳ���ձ�������������ȱ��500mL����ƿ����ͷ�ιܣ������������������������ҺŨ���к�Ӱ�죿���ƫ�ߡ�����ƫ�͡�������Ӱ�죩
A��Ũ���ᾭϡ�ͺ�δ��ȴ�����¾�ת������ƿ��ƫ�ߣ�
B������ƿδ��ɣ��ײ�������ˮ��Ӱ�죮
C������ʱ���ӿ̶��ߣ�ƫ�ͣ�
���� ��1������450mol•L-1��ϡ���ᣬӦѡ��500mL����ƿ��������Һϡ��ǰ�����ʵ����ʵ������������ҪŨ�����������ݴ�ѡ����ʹ�����Ͳ��
��2����������һ�����ʵ���Ũ����Һ�IJ�������ѡȡʵ�������������������������ʵ����ʵ�������Һ�������Ӱ�죬����C=$\frac{n}{V}$������������
��� �⣺��1��ʵ������10mol•L-1��Ũ��������450mol•L-1��ϡ���ᣬӦѡ��500mL����ƿ������ҪŨ�������ΪV��������Һϡ��ǰ�����ʵ����ʵ������䣺V��10mol•L-1=500mL��1mol•L-1�����V=50.0mL������Ӧѡ��50mL������Ͳ��
�ʴ�Ϊ��50.0��50��
��2������һ�����ʵ���Ũ����Һ�IJ������裺���㡢�������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȵȣ��õ��������У���ƽ���ձ�����������500mL����ƿ����ͷ�ιܣ����Ի�ȱ�ٵIJ�������Ϊ��500mL����ƿ����ͷ�ιܣ�
A��Ũ���ᾭϡ�ͺ�δ��ȴ�����¾�ת������ƿ�����ݺ���ȴ�����£���Һ���ƫС����Һ��Ũ��ƫ�ߣ�
B������ƿδ��ɣ��ײ�������ˮ�������ʵ����ʵ�������Һ�������������Ӱ�죬��ҺŨ�Ȳ��䣻
C������ʱ���ӿ̶��ߣ�������Һ�����ƫ����ҺŨ��ƫ�ͣ�
�ʴ�Ϊ��500mL����ƿ����ͷ�ιܣ�ƫ�ߣ���Ӱ�죻ƫ�ͣ�
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƣ���Ϥ����ԭ�������ǽ���ؼ���ע������ƿ����Ͳ���ѡ������ݣ���Ŀ�ѶȲ���

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д��ٵ������ڣ��ڵڰ����ڣ�������Ԫ�أ��ܸ���Ԫ�أ��ݢ�A�壻�ޢ�A�壻�ߢ�B�壻���A�壮
| A�� | �٢ۢ� | B�� | �ڢۢ� | C�� | �٢� | D�� | �٢ܢ� |
| A�� | $\stackrel{+4}{Mn}$O2��$\stackrel{+2}{Mn}$SO4 | B�� | Al 2O 3��Al��OH�� 3 | C�� | $\stackrel{-1}{KI}$��K$\stackrel{+5}{I}$O3 | D�� | HNO 3��NO |
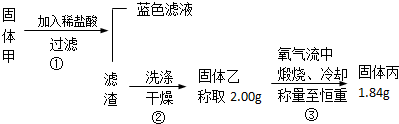
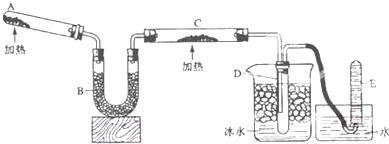
 ��ͼ��ʾװ�ã��ش��������⣺
��ͼ��ʾװ�ã��ش��������⣺