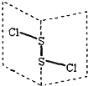
��Ŀ����
����Ŀ��I������7�ֻ�ѧ���ţ�18O��14C��23Na��14N��32S��16O��1H2
(1)��ʾ���صķ��Ź�______�֡�
(2)��Ϊͬλ�ص���______��______��
(3)��������ȣ������ܻ�Ϊͬλ�ص���______��______��
(4)��������ȣ�������������ȵ���______��______��
II��(1)д���������ʵĵ���ʽ�� NH3 _____________�� CO2______��
(2)2.2g笠�![]() ����������___�������������____��ij������һ��ԭ�Ӻˣ������� 17�����ӣ�20�����ӣ�������18�����ӣ������ӵĻ�ѧ������____��
����������___�������������____��ij������һ��ԭ�Ӻˣ������� 17�����ӣ�20�����ӣ�������18�����ӣ������ӵĻ�ѧ������____��
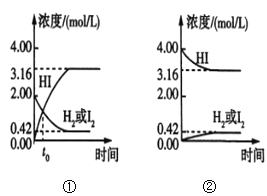
(3)��֪�Ͽ� 1mol H��H ����1mol I��I ����1mol H��I ���ֱ���Ҫ���յ�����Ϊ 436kJ��151kJ��299kJ������ 1mol H2�� 1mol I2���� 2mol HI��_____(���ų�������������)_____kJ ��������
(4)�����������ʣ���H2 ��Na2O2 ��NaOH ��H2O2 ��CaCl2 ��NH4NO3 ��H2S��ֻ�����Ӽ����ɵ�������_____(����ţ���ͬ)�������Ӽ��ͷǼ��Թ��ۼ����ɵ�������________�����ڹ��ۻ��������_____��
���𰸡�6 18O 16O 14C 14N 14C 16O ![]()
![]() 1.1NA NA
1.1NA NA ![]() �ų� 11 �� �� �ܢ�
�ų� 11 �� �� �ܢ�
��������
I�� (1)�����Ǿ���һ����Ŀ�����Ӻ�һ����Ŀ�����ӵ�һ��ԭ�ӣ��ʹ���6�ֺ��ء�
(2)ͬλ������������ͬ����������ͬ��ͬ��Ԫ�صIJ�ͬԭ�ӣ���Ϊͬλ�ص���18O��16O��
(3)���ط������Ͻǵ�����������������������ȣ������ܻ�Ϊͬλ�ص���14C��14N��
(4)������=������-��������14C��16O����������Ϊ8������������ȡ�
II��(1) NH3 ��CO2��Ϊ���ۻ��������ʽ�ֱ�Ϊ![]() ��
��![]() ��
��
(2)2.2g笠�![]() �����ʵ���Ϊ
�����ʵ���Ϊ![]() =0.1mol��1��
=0.1mol��1��![]() ��������Ϊ(14-7)+4(2-1)=11��������Ϊ7+4-1=10������2.2g笠�
��������Ϊ(14-7)+4(2-1)=11��������Ϊ7+4-1=10������2.2g笠�![]() ����������1.1NA�������������NA��ij������һ��ԭ�Ӻˣ������� 17�����ӣ�����ΪClԪ�أ���20�����ӣ���������Ϊ37��������18�����ӣ�����ΪCl-��������ӵĻ�ѧ������
����������1.1NA�������������NA��ij������һ��ԭ�Ӻˣ������� 17�����ӣ�����ΪClԪ�أ���20�����ӣ���������Ϊ37��������18�����ӣ�����ΪCl-��������ӵĻ�ѧ������![]() ��
��
(3) 1mol H2�� 1mol I2���� 2mol HI����Ҫ�Ͽ� 1mol H��H ����1mol I��I ��������436kJ+151kJ=587kJ������������2mol H��I ���ų�������Ϊ2��299kJ=598kJ������ 1mol H2�� 1mol I2���� 2mol HI��ų�598kJ-587kJ=11kJ ��������
(4)��H2���ɹ��ۼ��γɵĵ��ʣ�
��Na2O2����Na+��O22-��ͨ�����Ӽ����ɵ����ӻ����O22-��2����ԭ��֮��ͨ���Ǽ��Լ���ϣ�
��NaOH����Na+��OH-��ͨ�����Ӽ����ɵ����ӻ����OH-��O��H֮��ͨ�����Լ���ϣ�
��H2O2���ɼ��Լ��ͷǼ��Լ����ɵĹ��ۻ����
��CaCl2��Ca2+��Cl-ͨ�����Ӽ����ɵ����ӻ����
��NH4NO3��NH4+��NO3-ͨ�����Ӽ����ɵ����ӻ����NH4+��NO3-�����ɼ��Թ��ۼ����ɵ�ԭ���ţ�������NH4NO3�м������Ӽ������м��Թ��ۼ���
��H2S��H��Sͨ�����ۼ����ɵĹ��ۻ����
����ֻ�����Ӽ����ɵ������Ǣ�CaCl2�������Ӽ��ͷǼ��Թ��ۼ����ɵ������Ǣ�Na2O2�����ڹ��ۻ�������Ǣ�H2O2�͢�H2S��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ������ʵ��������ʵ���������ƥ�����
ʵ����� | ʵ������ | |
A | ��ʢ�и������������Һ���Թ���ͨ����������ϩ���� | ��Һ����ɫ����ȥ�����ú���Һ�ֲ� |
B | ��þ����ȼ��Ѹ�����뼯��CO2�ļ���ƿ | ����ƿ�в���Ũ�̲��к�ɫ�������� |
C | ��ʢ�б��������������Һ���Թ��еμ�ϡ���� | �д̼�����ζ�����������Һ����� |
D | ��ʢ��FeCl3��Һ���Թ��мӹ������ۣ�������1��KSCN��Һ | ��ɫ����ʧ����KSCN����Һ��ɫ���� |
A. AB. BC. CD. D